AA. Gastric C I 06. ppt - Università degli studi di Pavia
AA. Gastric C I 06. ppt - Università degli studi di Pavia
AA. Gastric C I 06. ppt - Università degli studi di Pavia
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Università</strong> <strong>degli</strong> Stu<strong>di</strong> <strong>di</strong> <strong>Pavia</strong><br />
<strong>AA</strong> 2005 - 2006<br />
Corso Integrato Geriatria ed Oncologia<br />
Insegnamento Oncologia Me<strong>di</strong>ca<br />
GASTRIC CANCER. I.<br />
Prof. Alberto Riccar<strong>di</strong>
GASTRIC ADENOCARCINOMA<br />
* incidence and mortality rates markedly ↓ over past 70 yrs;<br />
- 1930: gastric cancer → 1st cause of cancer - related<br />
deaths in American men (by a factor of 2) and 3rd cause in<br />
women (behind tumors of uterine cervix and breast);<br />
- in USA, mortality dropped in men from 28 to 5.0 and in<br />
women from 27 to 2.3 / 100,000 population (↓ worldwide);<br />
* nonetheless, in 1996, 22,800 new cases <strong>di</strong>agnosed and<br />
11,800 pts <strong>di</strong>ed;<br />
* incidence varies widely among <strong>di</strong>fferent countries, being<br />
comparatively high in Japan, China, Chile, and Ireland (with<br />
still decreased incidence and mortality)
GASTRIC ADENOCARCINOMA
GASTRIC ADENOCARCINOMA<br />
(Cancer Statistics, 2000, females)<br />
* age - adjusted death rates for gastric cancer in 2000 in 45 countries<br />
[no = per 100,000 population (rank among 45 countries)];<br />
- rates in USA, Canada and United Kingdom compared with selected<br />
countries (inclu<strong>di</strong>ng 15 countries with highest death rates)
GASTRIC ADENOCARCINOMA<br />
(Cancer Statistics, 2000, males)<br />
* age - adjusted death rates for gastric cancer in 2000 in 45 countries<br />
[no = per 100,000 population (rank among 45 countries)];<br />
- rates in USA, Canada and United Kingdom compared with selected<br />
countries (inclu<strong>di</strong>ng 15 countries with highest death rates)
* age - adjusted<br />
cancer death<br />
rates in USA from<br />
1930 to 1999 in<br />
selected sites:<br />
- steady ↓ in<br />
death rates for<br />
stomach, breast<br />
and colorectal<br />
cancers;<br />
- from 1960 to<br />
1998, continual ↑<br />
in death rates for<br />
lung cancer<br />
GASTRIC ADENOCARCINOMA<br />
(Cancer Statistics, females, 2003)<br />
stomach
* age - adjusted<br />
cancer death rates in<br />
United States from<br />
1930 to 1999 in<br />
selected sites; ;<br />
- similar ↓ in death<br />
rates from gastric<br />
cancer;<br />
- ↑ in death rates for<br />
lung cancer from 1930<br />
to 1990 with a<br />
continual decrease<br />
during the 1990s<br />
GASTRIC ADENOCARCINOMA<br />
(Cancer Statistics, males, 2003)<br />
stomach
GASTRIC ADENOCARCINOMA<br />
Epidemiology<br />
* an environmental exposure, beginning early<br />
in life, probably related to development of GC,<br />
with <strong>di</strong>etary carcinogens being most likely<br />
factor(s):<br />
- risk of GC greater among lower socio -<br />
economic classes;<br />
- migrants from high - to low - incidence<br />
nations maintain their susceptibility to GC (while<br />
risk for their offspring ~ that of new homeland)
RISK FACTORS AND PATHOGENESIS
GASTRIC ADENOCARCINOMA<br />
Risk factors. I.<br />
* <strong>di</strong>et (high in dry salted, smoked or preserved foods<br />
and low in fruits and vegetables);<br />
* bacteria (inclu<strong>di</strong>ng Helicobacter pylori infection);<br />
* advanced age;<br />
* male gender;<br />
* atrophic gastritis and intestinal metaplasia;<br />
* pernicious anemia;<br />
* cigarette smoking;<br />
* Menetrier's <strong>di</strong>sease (giant hypertrophic gastritis),<br />
and<br />
* gastric adenomatous polyps and familial polyposis
GASTRIC ADENOCARCINOMA<br />
Etiology. I. Diet and bacteria<br />
* relationship between <strong>di</strong>etary patterns and<br />
development of GC:<br />
- long - term ingestion of high concentrations<br />
of nitrates in dried, smoked and salted foods;<br />
- bacteria convert nitrates to carcinogenic<br />
nitrites (= nitrosamines)
Nitrate - converting bacteria as a factor in<br />
causation of gastric carcinoma
GASTRIC ADENOCARCINOMA<br />
Etiology. II. Bacteria and favoring growth factors<br />
* exogenous bacteria (= partially decayed foods,<br />
especially consumed by lower socioeconomic classes);<br />
- endogenous bacteria (such as Helicobacter pylori),<br />
whose growth results from loss of gastric aci<strong>di</strong>ty:<br />
* favoring factors:<br />
- partial gastrectomy to control benign peptic ulcer<br />
<strong>di</strong>sease (15 - 20 yrs earlier) abolishes acid - producing cells<br />
of gastric antrum;<br />
- achlorhydria, atrophic gastritis and pernicious anemia<br />
(= precancerous situations) in elderly (in pts with atrophic<br />
gastritis, usual gastric mucosa replaced by intestinal - type<br />
cells → intestinal metaplasia → cellular atypia and<br />
eventual neoplasia)
˚HELICOBACTER PYLORI IN A GASTRIC PIT
NATURAL HISTORY OF<br />
HELICOBACTER PYLORI<br />
INFECTION. I.<br />
* HP found worldwide (prevalence,<br />
up 100%, in developing countries;<br />
* HP products in stomach mucosa<br />
control aci<strong>di</strong>ty of environment and<br />
induce inflammation → several<br />
gastric <strong>di</strong>seases (weakening of<br />
mucosal barrier eventually result in<br />
ulceration);<br />
* major cause of chronic gastritis<br />
and associated with duodenal<br />
ulcer (90% of pts), gastric ulcer<br />
(70% of pts) and ?gastric cancer<br />
(?60% of pts)
NATURAL HISTORY OF HELICOBACTER PYLORI INFECTION. II.<br />
* most HP strains (= type<br />
I; e.g. strain 26695)<br />
causing <strong>di</strong>sease contain<br />
cag (= cytotoxin -<br />
associated Ag)<br />
chromosomal<br />
pathogenicity island (~<br />
37,000 bp and 29 genes);<br />
- cag island splits into 2<br />
parts, with most cag<br />
arranged genes involved<br />
in secretory machinery<br />
translocating protein<br />
CagA (cytotoxin -<br />
associated Ag A) into<br />
cytoplasm of gastric<br />
epithelial cells;<br />
* most genes involved in 2 major processes:<br />
- translocation of CagA from bacterium to host cell<br />
- production of IL - 8 (chemotactic for neutrophils<br />
and lymphocytes and angiogenic) by gastric cells
NATURAL HISTORY OF HELICOBACTER PYLORI INFECTION. III.<br />
* HP usually acquired in<br />
childhood;<br />
* acute HP infection (gray) →<br />
transient, rarely <strong>di</strong>agnosed hypo -<br />
chlorhydria;<br />
- persistent infection → chronic<br />
gastritis (green) in most pts (80 -<br />
90%, without symptoms);<br />
* variable further clinical course:<br />
hypocloridria<br />
- pts with ↑ acid output (red) →<br />
antral gastritis pre<strong>di</strong>sposing to<br />
duodenal ulcer;<br />
- pts with ↓ acid output (black) →<br />
gastritis in stomach’s body<br />
pre<strong>di</strong>sposing to gastric ulcer and (more rarely) to gastric carcinoma;<br />
[* HP infection induces mucosa - associated lymphoid tissue (MALT),<br />
possibly lea<strong>di</strong>ng to malignant lymphoma]
Pathogen - host interactions in<br />
pathogenesis of HP infection. I.<br />
* integral role of host response to<br />
HP in induction of damage to<br />
gastric epithelium:<br />
- during early phase of infection,<br />
bin<strong>di</strong>ng of HP to epithelial cells (EC)<br />
(especially by BabA and by strains<br />
harboring cag pathogenicity island)<br />
→ production of IL - 8 and other<br />
chemokines by EC [e.g., epithelial -<br />
cell - derived neutrophil - activating<br />
peptide 78 (ENA - 78) and growth -<br />
related oncogene (GRO - α)];<br />
- nuclear factor - κB (NF - κB) and<br />
early - response transcription -<br />
factor activator protein 1 (AP - 1) =<br />
intracellular messengers involved in<br />
this process
Pathogen - host interactions in<br />
pathogenesis of HP Infection. II.<br />
* chemokines secreted by<br />
epithelial cells bind to proteoglycan<br />
scaffol<strong>di</strong>ng → gra<strong>di</strong>ent by<br />
polymorphonuclear cells (PMN) are<br />
recruited;<br />
* chronic phase of HP gastritis<br />
associates an adaptive lymphocyte<br />
response with initial innate<br />
response;<br />
[- lymphocyte recruitment<br />
facilitated by chemokine -<br />
me<strong>di</strong>ated expression of vascular<br />
addressins [e.g., vascular - cell<br />
adhesion molecule 1 (VCAM - 1)]<br />
and intercellular adhesion molecule<br />
1 (ICAM - 1), required for<br />
lymphocyte extravasation]
Pathogen - host interactions in<br />
pathogenesis of HP Infection. III.<br />
* macrophages participating in IL -<br />
8 production → proinflammatory<br />
cytokines involved in activation of<br />
recruited cells [especially T helper<br />
cells (Th0, Th1, Th2), that respond<br />
with a biased Th1 response to HP;<br />
- in turn, Th1 - type cytokines such<br />
as interferon - γ (INF - γ) →<br />
expression of class II major<br />
histocompatibility complexes<br />
(MHC) and accessory molecules B7<br />
- 1 and B7 - 2 by epithelial cells →<br />
epithelial cells competent for<br />
antigen presentation
Pathogen - host interactions in<br />
pathogenesis of HP infection. IV.<br />
* tumor necrosis factor - α (TNF- α) (red)<br />
↑ cytotoxin VacA- and Fas- me<strong>di</strong>ated<br />
apoptosis induced by → <strong>di</strong>sruption of<br />
epithelial barrier → facilitation of<br />
translocation of bacterial antigens →<br />
further activation of macrophages<br />
(cytokines produced by macrophages<br />
also alter mucus secretion → HP -<br />
me<strong>di</strong>ated <strong>di</strong>sruption of mucous layer);<br />
* cytokines produced in gastric mucosa<br />
(green) → changes in gastric - acid<br />
secretion / homeostasis (dashed lines);<br />
* TNF - α, IL - 1β and IFN - γ ↑ gastrin<br />
release, stimulating parietal and<br />
enterochromaffin cells and thus acid<br />
secretion;<br />
* TNF- α also induces ↓ no. of antral D cells → decreased somatostatin production<br />
= in<strong>di</strong>rectly ↑ acid production (LPS = lipopolysaccharide)
Potential outcomes<br />
of sequencing<br />
of Helicobacter pylori genome
GASTRIC ADENOCARCINOMA<br />
Etiology. III. Other factors<br />
* gastric ulcers and adenomatous polyps (but<br />
unconvincing data on a cause / effect relationship = e.g.,<br />
inadequate clinical <strong>di</strong>stinction between benign gastric<br />
ulcers and small ulcerating GC);<br />
- Menetrier's <strong>di</strong>sease (extreme hypertrophy of gastric<br />
rugal folds mimics polypoid lesions, but does not represent<br />
true adenomatous polyps);<br />
- blood group A have ↑ incidence of GC than blood<br />
group 0 in<strong>di</strong>viduals (possibly due to # in mucous secretion<br />
of various ABO groups → greater or lesser mucosal<br />
protection from carcinogens);<br />
- no association between duodenal ulcers and GC
PATHOLOGY
GASTRIC CANCER<br />
Pathology. I.<br />
* 85% = adenocarcinomas (<strong>di</strong>ffuse and<br />
intestinal type);<br />
* 15% = non - Hodgkin's lymphomas and<br />
gatrointestinal stromal tumors (GIST, formerly<br />
called “leiomyosarcomas”)
GASTRIC CANCER<br />
Pathology. II.<br />
Polyps
GASTRIC CANCER<br />
Pathology. III.<br />
Leiomyoma<br />
(gastrointestinal<br />
stromal cell tumor,<br />
GIST?)
GASTRIC ADENOCARCINOMA<br />
Pathology. IV. Carcinoma, <strong>di</strong>ffuse type<br />
* cell cohesion absent, with in<strong>di</strong>vidual cells<br />
infiltrating and thickening stomach wall without<br />
forming a <strong>di</strong>screte mass;<br />
- more often in younger pts, develops<br />
throughout stomach, inclu<strong>di</strong>ng car<strong>di</strong>as → loss of<br />
<strong>di</strong>stensibility of gastric wall (“linitis plastica” or<br />
"leather bottle" appearance), with a far more<br />
ominous prognosis
GASTRIC ADENOCARCINOMA<br />
Pathology. V. Carcinoma, <strong>di</strong>ffuse type<br />
* right: low power view, with poorly<br />
<strong>di</strong>fferentiated cancer arising from mucosa<br />
and <strong>di</strong>ffusely infiltrating all layers of gastric<br />
wall;<br />
* left: ↑ magnification, with effacement of<br />
lamina propria of gastric mucosa
GASTRIC ADENOCARCINOMA<br />
Pathology. VI. Carcinoma, <strong>di</strong>ffuse type<br />
* linitis plastica carcinoma <strong>di</strong>ffusely infiltrates entire gastric wall<br />
without forming an intraluminal mass;<br />
* wall typically thickened ~ 2 - 3 cm, with leathery, inelastic<br />
consistency
GASTRIC<br />
ADENOCARCINOMA<br />
Pathology. VII.<br />
Carcinoma, <strong>di</strong>ffuse type<br />
Linitis plastica<br />
(scirrhous)
GASTRIC ADENOCARCINOMA<br />
Pathology. VIII. Carcinoma, <strong>di</strong>ffuse type
GASTRIC ADENOCARCINOMA<br />
Pathology. IX. Carcinoma, <strong>di</strong>ffuse type
GASTRIC ADENOCARCINOMA<br />
Pathology. X. Carcinoma, intestinal type<br />
* cohesive neoplastic cells forming gland -<br />
like tubular structures:<br />
- frequently ulcerative;<br />
- more commonly in antrum - prepylorus,<br />
car<strong>di</strong>a - fundus and lesser curvature of<br />
stomach, and<br />
- often preceeded by prolonged pre -<br />
cancerous process
GASTRIC ADENOCARCINOMA<br />
Pathology. XI. Carcinoma, signet ring cells
GASTRIC ADENOCARCINOMA<br />
Pathology. XII. Neuroendocrine carcinoma<br />
typical light and electron microscopy of carcinoid tumor, with<br />
numerous, fairly uniformly sized neurosecretory granules
GASTRIC<br />
ADENOCARCINOMA<br />
Pathology. XIII. Borrmann<br />
gross classification<br />
I = polypoid;<br />
II = ulcerating;<br />
III = ulcerating -<br />
infiltrating;<br />
IV = infiltrating
GASTRIC ADENOCARCINOMA<br />
Pathology. XIV. Ulcerating carcinoma
GASTRIC<br />
ADENOCARCINOMA<br />
Pathology. XV.<br />
Ulcerating<br />
carcinoma
GASTRIC ADENOCARCINOMA<br />
Pathology. XVI. Polypoid carcinoma
GASTRIC<br />
ADENOCARCINOMA<br />
Pathology. XVII.<br />
Polypoid carcinoma<br />
Carcinoma of car<strong>di</strong>a<br />
Carcinoma of fundus
GASTRIC<br />
ADENOCARCINOMA<br />
Pathology. XVIII.<br />
Polypoid carcinoma<br />
Carcinoma of antrus
GASTRIC ADENOCARCINOMA<br />
Pathology. XIX. Topography<br />
antrum and prepylorus ~ 30%<br />
car<strong>di</strong>a and fundus ~ 35%<br />
lesser curvature ~ 20%<br />
greater curvature ~ 3 - 5%<br />
entire stomach ~ 5 - 10%
GASTRIC ADENOCARCINOMA<br />
Pathology. XX.<br />
* ↓ incidence of cancer of <strong>di</strong>stal half of<br />
stomach (especially intestinal type);<br />
- no ↓ in <strong>di</strong>ffuse type GC (10% of pts);<br />
* cancer of car<strong>di</strong>a and gastroesophageal<br />
junction rising (dramatically under 40 yrs, in<br />
last 2 decades)
“EARLY” GASTRIC CANCER
GASTRIC ADENOCARCINOMA<br />
Pathology. XXI. Early gastric cancer<br />
* early gastric cancer = depth of invasion limited<br />
to mucosa or submucosa, regardless of LN<br />
involvement (gross pathology: type I = polypoid;<br />
type IIa = elevated; type IIb = flat; type IIc =<br />
depressed; type III = excavated);<br />
* advanced gastric cancer = <strong>di</strong>sease penetrates<br />
muscolar layer, usually associated with<br />
contiguous or <strong>di</strong>stant spread [gross pathology<br />
(Borrmann classification): I = polypoid; III =<br />
ulcerating; III = ulcerating - infiltrating; IV =<br />
infiltrating]
GASTRIC ADENOCARCINOMA<br />
Pathology. XXII. Early gastric cancer<br />
* early GC → limited to mucosa and submucosa;<br />
* advanced GC → tumor penetrates beyond submucosa
GASTRIC ADENOCARCINOMA<br />
Pathology. XXIII. Early gastric cancer<br />
macroscopically<br />
<strong>di</strong>vided<br />
into three types
GASTRIC ADENOCARCINOMA<br />
Pathology. XXIV.<br />
Early gastric cancer, elevated
GASTRIC<br />
ADENOCARCINOMA<br />
Pathology. XXV.<br />
Early gastric cancer,<br />
excavated
GASTRIC ADENOCARCINOMA<br />
Pathology. XXVI. Is early gastric cancer an<br />
“early” stage of advanced gastric cancer<br />
* ?early gastric cancer could be a <strong>di</strong>fferent<br />
<strong>di</strong>sease than advanced gastric cancer (because<br />
an inability to invade);<br />
* observation in<strong>di</strong>cating that early gastric cancer<br />
becomes advanced gastric cancer:<br />
- retrospective analysis of sequential X rays;<br />
- pts who refused surgery for early gastric cancer<br />
and developed advanced cancer;<br />
- animal <strong>stu<strong>di</strong></strong>es
GASTRIC ADENOCARCINOMA<br />
Pathology. XXVII.<br />
* % of pts with GC with lymph node involvement<br />
↑ as depth of invasion ↑ from mucosa to serosa;<br />
* 5 - yr survival ↓ as depth of invasion ↑
GENETICS
GASTRIC ADENOCARCINOMA<br />
Genetics. I.<br />
* incidence of <strong>di</strong>ffuse GC similar in most<br />
populations;<br />
- intestinal GC predominates in high - risk<br />
geographic regions (accounting for declining of<br />
GC);<br />
* <strong>di</strong>fferent etiologic factor(s) involved in these<br />
two subtypes?
GASTRIC ADENOCARCINOMA<br />
Genetics. II.<br />
* multiple genetic and epigenetic alterations<br />
in oncogenes, tumour - suppressor genes, cell -<br />
cycle regulators, cell adhesion molecules, DNA<br />
repair genes, genetic instability and telomerase<br />
activation implicated in multistep process of<br />
human stomach carcinogenesis;<br />
- combinations of alterations # in two<br />
histological types of GC → <strong>di</strong>stinct<br />
carcinogenetic pathways in well - <strong>di</strong>fferentiated<br />
(intestinal - type GC) and poorly <strong>di</strong>fferentiated<br />
(<strong>di</strong>ffuse - type GC)
GENETIC STEPS<br />
OF GASTRIC ADENOCARCINOMA
GENETIC STEPS OF INTESTINAL TYPE<br />
GASTRIC ADENOCARCINOMA
GENETIC STEPS OF DIFFUSE TYPE<br />
GASTRIC ADENOCARCINOMA<br />
Diffuse type
GASTRIC ADENOCARCINOMA<br />
Genetics. III. Intestinal - type GC<br />
* in multistep process of intestinal - type<br />
carcinogenesis, genetic pathway <strong>di</strong>vided into 3<br />
subpathways:<br />
- intestinal metaplasia → adenoma →<br />
carcinoma;<br />
- intestinal metaplasia → carcinoma, and<br />
- de novo;<br />
* infection with HP is a strong trigger for<br />
hyperplasia of hTERT+ (human telomerase reverse<br />
transcriptase, regulating human telomers and<br />
senescence) “stem cells” in intestinal metaplasia
Telomeres and telomerase<br />
* telomere = extension of DNA at chr ends, generated by<br />
telomerase reverse transcriptase (TERT, which uses an internally<br />
bound RNA loop as a template);<br />
[usually, “Hayflick limit” of ~ 50 - 70 <strong>di</strong>vision cycles for human<br />
<strong>di</strong>ploid cells me<strong>di</strong>ated by telomere length]
Schematic structure of telomere - repair complex<br />
and location of mutations<br />
* TERC, TERT,<br />
dyskerin, NOP10,<br />
NHP2 and GAR1<br />
constitute<br />
telomerase ribo -<br />
nucleoprotein<br />
complex;<br />
* mutations of<br />
amino acids<br />
denoted by their<br />
single - letter<br />
codes<br />
Yamaguchi H et al N Engl J Med 2005; 352: 1413 - 24
GASTRIC ADENOCARCINOMA<br />
Genetics. IV. Intestinal - type GC<br />
* infection with HP is<br />
a strong trigger for<br />
hyperplasia of hTERT+<br />
(Human Telomerase<br />
Reverse Transcriptase,<br />
regulating human<br />
telomers and cell<br />
senescence) “stem<br />
cells” in intestinal<br />
metaplasia →<br />
adenoma →<br />
carcinoma sequence;<br />
* genetic instability and hyperplasia of hTERT+ “stem cells”→ replication<br />
error (at D1S191 locus), DNA hypermethylation (D17S5 locus), pS2 loss,<br />
RARβ loss, CD44 abnormal transcripts and p53 mutation;<br />
- all these epi- and genetic alterations in > 30% of incomplete intestinal<br />
metaplasias and common in intestinal - type GC
* adenoma →<br />
carcinoma sequence<br />
in ~ 20% of gastric<br />
adenomas, with<br />
ad<strong>di</strong>tional events<br />
inclu<strong>di</strong>ng APC and p53<br />
mutations, loss of<br />
heterozygosity (LOH),<br />
reduced p27 and<br />
cyclin E expression<br />
and presence of c -<br />
met 6.0 - kb transcripts;<br />
GASTRIC ADENOCARCINOMA<br />
Genetics. V. Intestinal - type GC<br />
* advanced intestinal - type gastric cancer associated with further<br />
events (inclu<strong>di</strong>ng c - erbB gene amplification, DCC loss, 1q LOH, p27 loss,<br />
reduced tumour growth factor (TGF) - β type I receptor expression and<br />
reduced nm23 expression)
GASTRIC ADENOCARCINOMA<br />
Genetics. V. Intestinal - type GC<br />
* de - novo pathway for carcinogenesis of<br />
well - <strong>di</strong>fferentiated GC involves LOH and<br />
abnormal expression of p73 gene (responsible<br />
for development of foveolar - type gastric<br />
cancers with pS2 expression)
GASTRIC ADENOCARCINOMA<br />
Genetics. VI. Diffuse - type GC<br />
* loss of E - cadherin, LOH at chr 17p and mutation or LOH of<br />
p53 preferentially involved in development of poorly<br />
<strong>di</strong>fferentiated GC
ROLE OF CADHERINS IN ESTABLISHING MOLECULAR LINKS<br />
BETWEEN ADJACENT CELLS<br />
* cadherin <strong>di</strong>mers<br />
gather at adherens<br />
junctions to form a<br />
zipper - like structure<br />
that maintains<br />
adjacent cells in close<br />
contact;<br />
* cadherins linked to<br />
cytoskeleton through<br />
cytoplasmic catenins<br />
(essential for normal<br />
cadherin function and<br />
formation of adherens<br />
junctions);<br />
- cadherin linked to β - catenin (or related plakoglobin) and α -<br />
catenin associated with actin microfilaments
GASTRIC ADENOCARCINOMA<br />
Genetics. VII. Diffuse - type GC,<br />
Here<strong>di</strong>tary forms<br />
* germline mutations in E - cadherin (CDH1)<br />
gene (on chr 16, inherited in autosomal<br />
dominant pattern and co<strong>di</strong>ng for cell<br />
adhesion proteins) linked to high incidence<br />
of occult gastric cancers in young<br />
asymptomatic carriers → ~ 70% lifetime risk<br />
for here<strong>di</strong>tary <strong>di</strong>ffuse gastric adeno -<br />
carcinoma
Model for development of<br />
here<strong>di</strong>tary <strong>di</strong>ffuse gastric cancer<br />
* gastric mucosa in CDH1 (E - cadherin<br />
gene) germline mutation carriers is<br />
normal until second CDH1 allele is<br />
inactivated or repressed (second hit) by<br />
transcriptional downregulation;<br />
[- promoter hypermethylation is one<br />
mechanism for downregulation<br />
(transcription factor me<strong>di</strong>ated events<br />
also play a role, inclu<strong>di</strong>ng environmental<br />
and physiological factors such as <strong>di</strong>et,<br />
carcinogen exposure, ulceration and<br />
gastritis)];<br />
- since downregulation may occur in<br />
multiple cells, multifocal tumors develop;<br />
- tumor expands slowly until ad<strong>di</strong>tional genetic events, possibly combined with<br />
an altered microenvironment → clonal expansion and <strong>di</strong>sease progression;<br />
[- because second hit does not involve somatic, irreversible, mutation of second<br />
CDH1 allele, it is possible that early stage lesions is reversible]
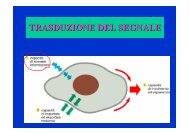
RECEPTOR TYROSINE KINASE AND PROLIFERATION AND TISSUE EXPANSION<br />
* receptor tyrosine<br />
kinases (RTKs) = main<br />
positive regulators of<br />
proliferation and tissue<br />
expansion (through<br />
downstream pathways<br />
inclu<strong>di</strong>ng ras - MAPK and<br />
PI3K / Akt cascade);<br />
* RTKs negatively act on<br />
E - cadherin function →<br />
<strong>di</strong>sassembly of adherens<br />
junctions;<br />
- downstream elements of RTK signalling interact and activate Rho family<br />
GTPases (Rac, Rho, Cdc42) system that interfere with cadherin - me<strong>di</strong>ated<br />
adhesion through changes in actin cytoskeleton;<br />
* catenin p120 acts as regulator of cadherin function either promoting or<br />
repressing E - cadherin - dependent adhesion
Pe<strong>di</strong>grees fulfilling<br />
Here<strong>di</strong>tary Diffuse <strong>Gastric</strong> Cancer (HDGC) criteria<br />
(examples)<br />
* ≥ 2 pathologically documented cases of<br />
<strong>di</strong>ffuse gastric cancer in 1st- or 2nd- degree<br />
relatives, with ≥ 1 <strong>di</strong>agnosed at < 50 yrs;<br />
* ≥ 3 pathologically documented cases of<br />
<strong>di</strong>ffuse gastric cancer in 1st- or 2nd - degree<br />
relatives of any age
SCHEMA TO GUIDE THE MANAGEMENT<br />
OF FAMILIAL GASTRIC CANCER KINDREDS
FAMILY WITH PREDICTIVE GENETIC - TESTING (A)<br />
AND SEQUENCE CHROMATOGRAM OF CDH1 (EXON 12) (B)<br />
A) squares = male and circles = female members;<br />
- = unaffected and = affected persons;<br />
- slash = death and line under symbol = prophylactic gastrectomy;<br />
- + sign = mutation+, - sign = mutation-, in parentheses = result of pre<strong>di</strong>ctive testing;<br />
- arrow identifies proband [age at <strong>di</strong>agnosis and death (in parentheses) under each<br />
symbol];<br />
B) codon 598 in yellow;<br />
- pt IV - 2 = wild - type sequence and pt IV - 1 = heterozygous for C2095T mutation;<br />
[- Opa = opal nonsense mutation (one of 3 nonsense codons pre<strong>di</strong>cting protein<br />
truncation) (NL = normal sequence and Mut = mutation; N in nucleotide chromatogram<br />
in<strong>di</strong>cates that both C and T are present)]
GASTRIC ADENOCARCINOMA<br />
Genetics. VIII.<br />
* from prophylactic gastrectomy in pts to<br />
regular endoscopic surveillance with multiple<br />
random biopsies → presence of microscopic<br />
intrahepitelial carcinomas → early total<br />
gastrectomy for this small pt’s population (lack<br />
of other effective early tumor detection with less<br />
aggressive approaches)
Early <strong>di</strong>ffuse gastric cancers<br />
from prophylactic - gastrectomy<br />
specimens<br />
A) superficial infiltrate of signet - ring<br />
carcinoma cells (EE staining);<br />
B) immunohistochemistry with Abs to<br />
type IV collagen = invasive nature of<br />
signet - cell infiltrates (thick<br />
basement membrane under surface<br />
epithelium and around both glands and<br />
capillaries stains strongly; no <strong>di</strong>stinct<br />
staining around signet - ring cells);<br />
C) signet - ring - cells infiltrate in<br />
superficial lamina propria in gastric<br />
car<strong>di</strong>a (PAS with <strong>di</strong>astase staining for<br />
mucina);<br />
D) epithelial nature of infiltrate<br />
(immunohistochemistry for cytokeratin);<br />
E) in situ signet - ring - cell lesions in<br />
gastric car<strong>di</strong>a;<br />
F, G, H) early <strong>di</strong>ffuse gastric cancers<br />
(EE)
CLINICAL FEATURES
GASTRIC ADENOCARCINOMA<br />
Clinical features. I.<br />
* usually no symptoms when superficial and surgically<br />
curable;<br />
* with more extensive tumors → insi<strong>di</strong>ous upper<br />
abdominal <strong>di</strong>scomfort (from a vague, postpran<strong>di</strong>al<br />
fullness to severe, steady pain), anorexia (often with slight<br />
nausea) and weight loss;<br />
* no early physical sign (fin<strong>di</strong>ng of a palpable<br />
abdominal mass generally in<strong>di</strong>cates long - stan<strong>di</strong>ng<br />
growth and regional extension)
GASTRIC ADENOCARCINOMA<br />
Clinical features. II.<br />
* possible early symptoms (with superficial and<br />
surgically curable tumors);<br />
- nausea and vomiting (with tumors of pylorus);<br />
- dysphagia (with lesions of car<strong>di</strong>a);
GASTRIC ADENOCARCINOMA<br />
Clinical features. III.<br />
* iron - deficiency anemia in men and of<br />
occult blood in stool in both sexes → search for<br />
an occult lesion in gastrointestinal tract<br />
(especially in pts with atrophic gastritis or<br />
pernicious anemia);<br />
* unusual clinical features:<br />
- migratory thrombophlebitis;<br />
- microangiopathic hemolytic anemia, and<br />
- acanthosis nigricans
GASTRIC ADENOCARCINOMA<br />
Clinical features. IV. Acanthosis nigricans
GASTRIC ADENOCARCINOMA<br />
Clinical features. V. Acanthosis nigricans
GASTRIC<br />
ADENOCARCINOMA<br />
Clinical features. VI.<br />
Acanthosis nigricans