DNA triplex structures in neurodegenerative disorder, Friedreich's ...
DNA triplex structures in neurodegenerative disorder, Friedreich's ...
DNA triplex structures in neurodegenerative disorder, Friedreich's ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
522 Moganty R Rajeswari<br />
with successive generations. Of the 10 to 12 TREs known till<br />
date, most are rich <strong>in</strong> G and C content (Warren 1996) and<br />
belong to the type (CXG)n, where X=is A, G, C or T .<br />
Further, triplet repeats are found <strong>in</strong> every region of the gene<br />
like <strong>in</strong>trons, exons and the 3′ or 5′ untranslated regions<br />
(UTR) (Wilmot and Warren 1998). Genes of healthy humans<br />
also do conta<strong>in</strong> <strong>DNA</strong> triplet repeats of short length and their<br />
number is dist<strong>in</strong>ctly small. However, when the number of<br />
repeats reaches a certa<strong>in</strong> value, the symptoms of the disease<br />
start manifest<strong>in</strong>g. For full-blown diseases the repeat number<br />
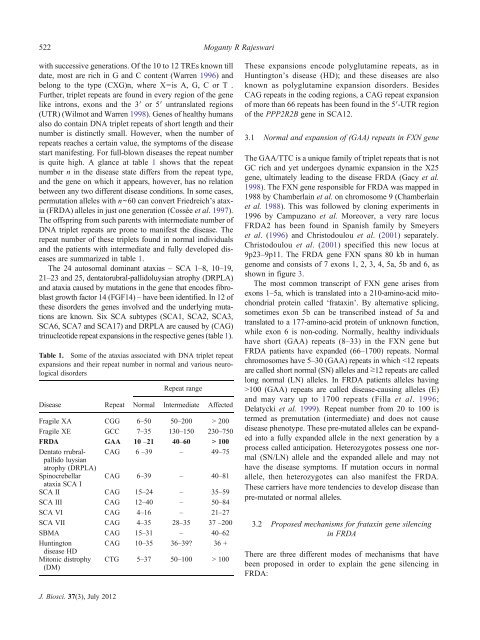
is quite high. A glance at table 1 shows that the repeat<br />
number n <strong>in</strong> the disease state differs from the repeat type,<br />
and the gene on which it appears, however, has no relation<br />
between any two different disease conditions. In some cases,<br />
permutation alleles with n~60 can convert Friedreich’s ataxia<br />
(FRDA) alleles <strong>in</strong> just one generation (Cossée et al. 1997).<br />
The offspr<strong>in</strong>g from such parents with <strong>in</strong>termediate number of<br />
<strong>DNA</strong> triplet repeats are prone to manifest the disease. The<br />
repeat number of these triplets found <strong>in</strong> normal <strong>in</strong>dividuals<br />
and the patients with <strong>in</strong>termediate and fully developed diseases<br />
are summarized <strong>in</strong> table 1.<br />
The 24 autosomal dom<strong>in</strong>ant ataxias – SCA 1–8, 10–19,<br />
21–23 and 25, dentatorubral-pallidoluysian atrophy (DRPLA)<br />
and ataxia caused by mutations <strong>in</strong> the gene that encodes fibroblast<br />
growth factor 14 (FGF14) – have been identified. In 12 of<br />
these <strong>disorder</strong>s the genes <strong>in</strong>volved and the underly<strong>in</strong>g mutations<br />
are known. Six SCA subtypes (SCA1, SCA2, SCA3,<br />
SCA6,SCA7andSCA17)andDRPLAarecausedby(CAG)<br />
tr<strong>in</strong>ucleotide repeat expansions <strong>in</strong> the respective genes (table 1).<br />
Table 1. Some of the ataxias associated with <strong>DNA</strong> triplet repeat<br />
expansions and their repeat number <strong>in</strong> normal and various neurological<br />
<strong>disorder</strong>s<br />
Disease<br />
Repeat<br />
Repeat range<br />
Normal Intermediate Affected<br />
Fragile XA CGG 6–50 50–200 > 200<br />
Fragile XE GCC 7–35 130–150 230–750<br />
FRDA GAA 10 –21 40–60 > 100<br />
Dentato rrubralpallido<br />
CAG 6 –39 – 49–75<br />
luysian<br />
atrophy (DRPLA)<br />
Sp<strong>in</strong>ocrebellar CAG 6–39 – 40–81<br />
ataxia SCA I<br />
SCA II CAG 15–24 – 35–59<br />
SCA III CAG 12–40 – 50–84<br />
SCA VI CAG 4–16 – 21–27<br />
SCA VII CAG 4–35 28–35 37 –200<br />
SBMA CAG 15–31 – 40–62<br />
Hunt<strong>in</strong>gton CAG 10–35 36–39? 36 +<br />
disease HD<br />
Mitonic distrophy<br />
(DM)<br />
CTG 5–37 50–100 > 100<br />
These expansions encode polyglutam<strong>in</strong>e repeats, as <strong>in</strong><br />
Hunt<strong>in</strong>gton’s disease (HD); and these diseases are also<br />
known as polyglutam<strong>in</strong>e expansion <strong>disorder</strong>s. Besides<br />
CAG repeats <strong>in</strong> the cod<strong>in</strong>g regions, a CAG repeat expansion<br />
of more than 66 repeats has been found <strong>in</strong> the 5′-UTR region<br />
of the PPP2R2B gene <strong>in</strong> SCA12.<br />
3.1 Normal and expansion of (GAA) repeats <strong>in</strong> FXN gene<br />
The GAA/TTC is a unique family of triplet repeats that is not<br />
GC rich and yet undergoes dynamic expansion <strong>in</strong> the X25<br />
gene, ultimately lead<strong>in</strong>g to the disease FRDA (Gacy et al.<br />
1998). The FXN gene responsible for FRDA was mapped <strong>in</strong><br />
1988 by Chamberla<strong>in</strong> et al. on chromosome 9 (Chamberla<strong>in</strong><br />
et al. 1988). This was followed by clon<strong>in</strong>g experiments <strong>in</strong><br />
1996 by Campuzano et al. Moreover, a very rare locus<br />
FRDA2 has been found <strong>in</strong> Spanish family by Smeyers<br />
et al. (1996) and Christodoulou et al. (2001) separately.<br />
Christodoulou et al. (2001) specified this new locus at<br />
9p23–9p11. The FRDA gene FXN spans 80 kb <strong>in</strong> human<br />
genome and consists of 7 exons 1, 2, 3, 4, 5a, 5b and 6, as<br />
shown <strong>in</strong> figure 3.<br />
The most common transcript of FXN gene arises from<br />
exons 1–5a, which is translated <strong>in</strong>to a 210-am<strong>in</strong>o-acid mitochondrial<br />
prote<strong>in</strong> called ‘fratax<strong>in</strong>’. By alternative splic<strong>in</strong>g,<br />
sometimes exon 5b can be transcribed <strong>in</strong>stead of 5a and<br />
translated to a 177-am<strong>in</strong>o-acid prote<strong>in</strong> of unknown function,<br />
while exon 6 is non-cod<strong>in</strong>g. Normally, healthy <strong>in</strong>dividuals<br />
have short (GAA) repeats (8–33) <strong>in</strong> the FXN gene but<br />
FRDA patients have expanded (66–1700) repeats. Normal<br />
chromosomes have 5–30 (GAA) repeats <strong>in</strong> which 100 (GAA) repeats are called disease-caus<strong>in</strong>g alleles (E)<br />
and may vary up to 1700 repeats (Filla et al. 1996;<br />
Delatycki et al. 1999). Repeat number from 20 to 100 is<br />
termed as premutation (<strong>in</strong>termediate) and does not cause<br />
disease phenotype. These pre-mutated alleles can be expanded<br />
<strong>in</strong>to a fully expanded allele <strong>in</strong> the next generation by a<br />
process called anticipation. Heterozygotes possess one normal<br />
(SN/LN) allele and the expanded allele and may not<br />
have the disease symptoms. If mutation occurs <strong>in</strong> normal<br />
allele, then heterozygotes can also manifest the FRDA.<br />
These carriers have more tendencies to develop disease than<br />
pre-mutated or normal alleles.<br />
3.2 Proposed mechanisms for fratax<strong>in</strong> gene silenc<strong>in</strong>g<br />
<strong>in</strong> FRDA<br />
There are three different modes of mechanisms that have<br />
been proposed <strong>in</strong> order to expla<strong>in</strong> the gene silenc<strong>in</strong>g <strong>in</strong><br />
FRDA:<br />
J. Biosci. 37(3), July 2012