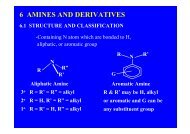
amine 1.1 Primary amine , 1o amine , RNH 1.2 Secondary amine ...
amine 1.1 Primary amine , 1o amine , RNH 1.2 Secondary amine ...
amine 1.1 Primary amine , 1o amine , RNH 1.2 Secondary amine ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ก Amines<br />
1. . salt formation<br />
(CH3)2NH + HNO3 (CH3)2NH2 + NO3 -<br />
NH2 + HCl<br />
NH3 + Cl-<br />
aromatic <strong>amine</strong> ก<br />
ก<br />
NH2<br />
G<br />
G = amino, alkoxide, , methyl <br />
G = nitro, cyano, sulfonic <br />
ก Amines<br />
2. . conversion into amide<br />
Ex:<br />
<strong>RNH</strong>2<br />
O<br />
RCCl<br />
O<br />
O<br />
RC-NHR<br />
O<br />
R2NH RCCl RCNR2<br />
O<br />
R3N<br />
RCCl No reaction<br />
NH2<br />
O<br />
CH3COH<br />
O<br />
NHCCH3<br />
+ H2O<br />
ก Amines<br />
3. . reaction with nitrous acid<br />
HNO 2<br />
ก Amines<br />
3. . reaction with nitrous acid<br />
1. <strong>Primary</strong> aromatic Diazonium salt<br />
2. <strong>Primary</strong> aliphatic <br />
<br />
3. Second aromatic and aliphatic<br />
ArNHR<br />
or<br />
R2NH<br />
NaNO2<br />
HCl<br />
R<br />
Ar-N-N=O<br />
or<br />
R2N-N=O<br />
N-Nitroso<strong>amine</strong><br />
4. Tertiary aromatic<br />
NR2<br />
HONO<br />
O=N<br />
NR2<br />
p-Nitroso compound