Physical bases of freezing point measurement using ... - Boschung
Physical bases of freezing point measurement using ... - Boschung
Physical bases of freezing point measurement using ... - Boschung
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Proceedings <strong>of</strong> the 9 th SIRWEC Conference 15-17 March 1998, Luleå, Sweden 280<br />
with m = m + m .<br />
tot NaCl H2O The volumetric properties <strong>of</strong> aqueous sodium chloride solutions are for instance given<br />
in [Rogers and Pitzer 1982]. For temperatures close to 0 °C, the specific volume ν (in cm 3 /g)<br />
can be fitted by :<br />
2<br />
ν( c) = 0. 653c − 0. 704 c+<br />
0. 999<br />
It is then possible to compute the thickness e <strong>of</strong> the solution :<br />
e =<br />
c ⋅ m<br />
A<br />
. (8)<br />
ν( ) tot . (9)<br />
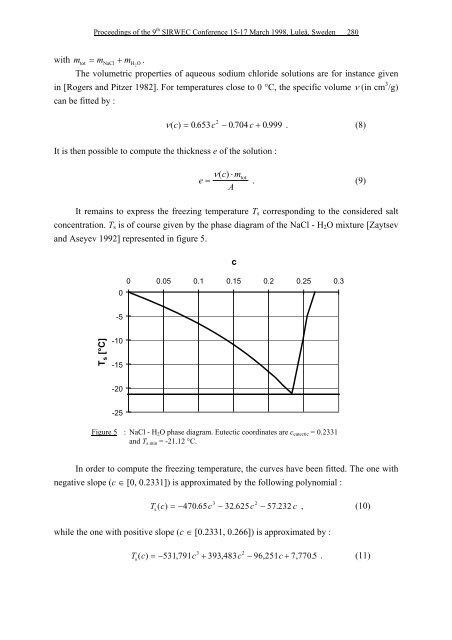
It remains to express the <strong>freezing</strong> temperature Ts corresponding to the considered salt<br />
concentration. Ts is <strong>of</strong> course given by the phase diagram <strong>of</strong> the NaCl - H2O mixture [Zaytsev<br />
and Aseyev 1992] represented in figure 5.<br />
T s [°C]<br />
0<br />
-5<br />
-10<br />
-15<br />
-20<br />
-25<br />
c<br />
0 0.05 0.1 0.15 0.2 0.25 0.3<br />
Figure 5 : NaCl - H2O phase diagram. Eutectic coordinates are ceutectic = 0.2331<br />
and Ts min = -21.12 °C.<br />
In order to compute the <strong>freezing</strong> temperature, the curves have been fitted. The one with<br />
negative slope (c ∈ [0, 0.2331]) is approximated by the following polynomial :<br />
3 2<br />
T ( c) =−470. 65c −32. 625c −57.<br />
232 c<br />
s<br />
while the one with positive slope (c ∈ [0.2331, 0.266]) is approximated by :<br />
, (10)<br />
3 2<br />
Ts( c) =− 531, 791c + 393, 483c − 96, 251c+ 7, 770. 5 . (11)