A Toy Model of Chemical Reaction Networks - TBI - Universität Wien
A Toy Model of Chemical Reaction Networks - TBI - Universität Wien
A Toy Model of Chemical Reaction Networks - TBI - Universität Wien
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3.2. FURTHER APPROXIMATIONS 19<br />
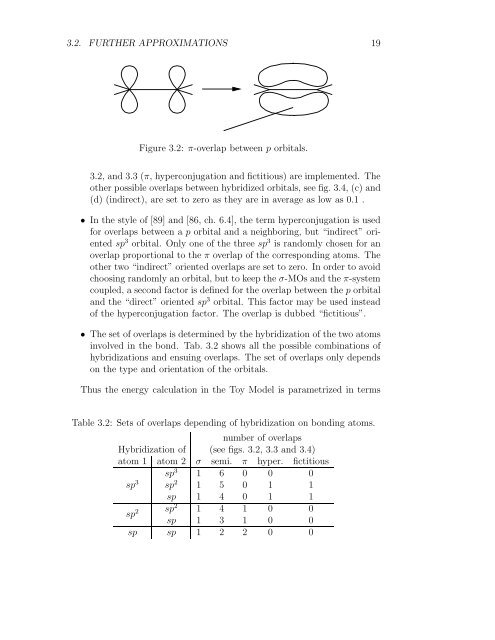
Figure 3.2: π-overlap between p orbitals.<br />
3.2, and 3.3 (π, hyperconjugation and fictitious) are implemented. The<br />
other possible overlaps between hybridized orbitals, see fig. 3.4, (c) and<br />
(d) (indirect), are set to zero as they are in average as low as 0.1 .<br />
• In the style <strong>of</strong> [89] and [86, ch. 6.4], the term hyperconjugation is used<br />
for overlaps between a p orbital and a neighboring, but “indirect” oriented<br />
sp 3 orbital. Only one <strong>of</strong> the three sp 3 is randomly chosen for an<br />
overlap proportional to the π overlap <strong>of</strong> the corresponding atoms. The<br />
other two “indirect” oriented overlaps are set to zero. In order to avoid<br />
choosing randomly an orbital, but to keep the σ-MOs and the π-system<br />
coupled, a second factor is defined for the overlap between the p orbital<br />
and the “direct” oriented sp 3 orbital. This factor may be used instead<br />
<strong>of</strong> the hyperconjugation factor. The overlap is dubbed “fictitious”.<br />
• The set <strong>of</strong> overlaps is determined by the hybridization <strong>of</strong> the two atoms<br />
involved in the bond. Tab. 3.2 shows all the possible combinations <strong>of</strong><br />
hybridizations and ensuing overlaps. The set <strong>of</strong> overlaps only depends<br />
on the type and orientation <strong>of</strong> the orbitals.<br />
Thus the energy calculation in the <strong>Toy</strong> <strong>Model</strong> is parametrized in terms<br />
Table 3.2: Sets <strong>of</strong> overlaps depending <strong>of</strong> hybridization on bonding atoms.<br />
number <strong>of</strong> overlaps<br />
Hybridization <strong>of</strong> (see figs. 3.2, 3.3 and 3.4)<br />
atom 1 atom 2 σ semi. π hyper. fictitious<br />
sp 3 1 6 0 0 0<br />
sp 3 sp 2 1 5 0 1 1<br />
sp 1 4 0 1 1<br />
sp 2 sp 2 1 4 1 0 0<br />
sp 1 3 1 0 0<br />
sp sp 1 2 2 0 0