A Toy Model of Chemical Reaction Networks - TBI - Universität Wien
A Toy Model of Chemical Reaction Networks - TBI - Universität Wien
A Toy Model of Chemical Reaction Networks - TBI - Universität Wien
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
50 CHAPTER 6. COMPUTATIONAL RESULTS<br />
O<br />
2.66<br />
O<br />
O<br />
O<br />
O<br />
1.44<br />
O<br />
O<br />
O<br />
O<br />
O<br />
2.66<br />
2.66<br />
O<br />
O<br />
O<br />
2.66<br />
O<br />
O<br />
O<br />
O<br />
O<br />
2.13<br />
1.44<br />
O<br />
O<br />
O<br />
2.66<br />
5.71<br />
O<br />
O<br />
O<br />
O<br />
1.44<br />
1.44<br />
O<br />
1.44<br />
O<br />
4.47<br />
2.66<br />
1.44<br />
O<br />
O<br />
O<br />
1.44<br />
O<br />
2.66<br />
O<br />
4.46<br />
4.47<br />
O<br />
O<br />
4.47<br />
4.46<br />
4.47<br />
O<br />
O<br />
O<br />
O<br />
2.66<br />
O<br />
4.46<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
1.44<br />
O<br />
O<br />
O<br />
O<br />
1.17<br />
1.17<br />
1.17<br />
O<br />
O<br />
4.46<br />
1.17<br />
1.17<br />
O<br />
O<br />
O<br />
1.33<br />
O<br />
O<br />
O<br />
O<br />
O<br />
3.37<br />
3.72<br />
O<br />
1.45<br />
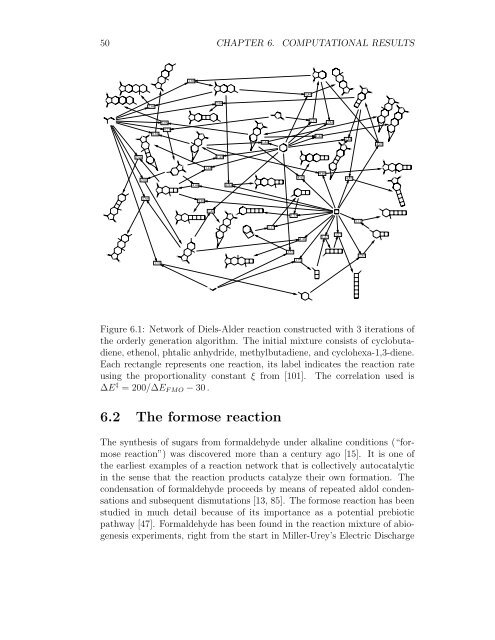
Figure 6.1: Network <strong>of</strong> Diels-Alder reaction constructed with 3 iterations <strong>of</strong><br />
the orderly generation algorithm. The initial mixture consists <strong>of</strong> cyclobutadiene,<br />
ethenol, phtalic anhydride, methylbutadiene, and cyclohexa-1,3-diene.<br />
Each rectangle represents one reaction, its label indicates the reaction rate<br />
using the proportionality constant ξ from [101]. The correlation used is<br />
∆E ‡ = 200/∆E FMO − 30.<br />
6.2 The formose reaction<br />
The synthesis <strong>of</strong> sugars from formaldehyde under alkaline conditions (“formose<br />
reaction”) was discovered more than a century ago [15]. It is one <strong>of</strong><br />
the earliest examples <strong>of</strong> a reaction network that is collectively autocatalytic<br />
in the sense that the reaction products catalyze their own formation. The<br />
condensation <strong>of</strong> formaldehyde proceeds by means <strong>of</strong> repeated aldol condensations<br />
and subsequent dismutations [13, 85]. The formose reaction has been<br />
studied in much detail because <strong>of</strong> its importance as a potential prebiotic<br />
pathway [47]. Formaldehyde has been found in the reaction mixture <strong>of</strong> abiogenesis<br />
experiments, right from the start in Miller-Urey’s Electric Discharge