Lecture Notes on Compositional Data Analysis - Sedimentology ...
Lecture Notes on Compositional Data Analysis - Sedimentology ...
Lecture Notes on Compositional Data Analysis - Sedimentology ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
42 Chapter 5. Exploratory data analysis<br />
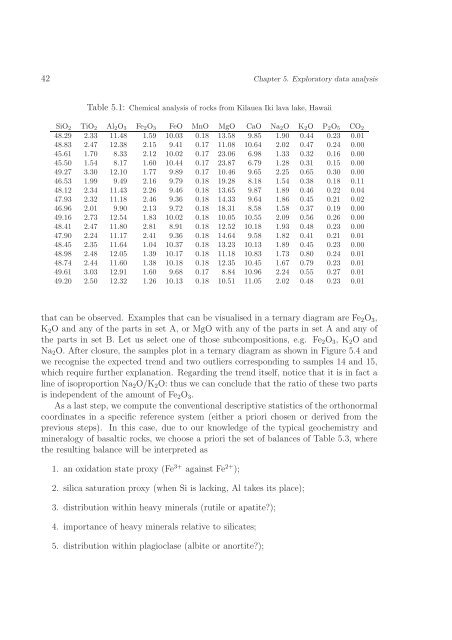
Table 5.1: Chemical analysis of rocks from Kilauea Iki lava lake, Hawaii<br />
SiO 2 TiO 2 Al 2 O 3 Fe 2 O 3 FeO MnO MgO CaO Na 2 O K 2 O P 2 O 5 CO 2<br />
48.29 2.33 11.48 1.59 10.03 0.18 13.58 9.85 1.90 0.44 0.23 0.01<br />
48.83 2.47 12.38 2.15 9.41 0.17 11.08 10.64 2.02 0.47 0.24 0.00<br />
45.61 1.70 8.33 2.12 10.02 0.17 23.06 6.98 1.33 0.32 0.16 0.00<br />
45.50 1.54 8.17 1.60 10.44 0.17 23.87 6.79 1.28 0.31 0.15 0.00<br />
49.27 3.30 12.10 1.77 9.89 0.17 10.46 9.65 2.25 0.65 0.30 0.00<br />
46.53 1.99 9.49 2.16 9.79 0.18 19.28 8.18 1.54 0.38 0.18 0.11<br />
48.12 2.34 11.43 2.26 9.46 0.18 13.65 9.87 1.89 0.46 0.22 0.04<br />
47.93 2.32 11.18 2.46 9.36 0.18 14.33 9.64 1.86 0.45 0.21 0.02<br />
46.96 2.01 9.90 2.13 9.72 0.18 18.31 8.58 1.58 0.37 0.19 0.00<br />
49.16 2.73 12.54 1.83 10.02 0.18 10.05 10.55 2.09 0.56 0.26 0.00<br />
48.41 2.47 11.80 2.81 8.91 0.18 12.52 10.18 1.93 0.48 0.23 0.00<br />
47.90 2.24 11.17 2.41 9.36 0.18 14.64 9.58 1.82 0.41 0.21 0.01<br />
48.45 2.35 11.64 1.04 10.37 0.18 13.23 10.13 1.89 0.45 0.23 0.00<br />
48.98 2.48 12.05 1.39 10.17 0.18 11.18 10.83 1.73 0.80 0.24 0.01<br />
48.74 2.44 11.60 1.38 10.18 0.18 12.35 10.45 1.67 0.79 0.23 0.01<br />
49.61 3.03 12.91 1.60 9.68 0.17 8.84 10.96 2.24 0.55 0.27 0.01<br />
49.20 2.50 12.32 1.26 10.13 0.18 10.51 11.05 2.02 0.48 0.23 0.01<br />
that can be observed. Examples that can be visualised in a ternary diagram are Fe 2 O 3 ,<br />
K 2 O and any of the parts in set A, or MgO with any of the parts in set A and any of<br />
the parts in set B. Let us select <strong>on</strong>e of those subcompositi<strong>on</strong>s, e.g. Fe 2 O 3 , K 2 O and<br />
Na 2 O. After closure, the samples plot in a ternary diagram as shown in Figure 5.4 and<br />
we recognise the expected trend and two outliers corresp<strong>on</strong>ding to samples 14 and 15,<br />
which require further explanati<strong>on</strong>. Regarding the trend itself, notice that it is in fact a<br />
line of isoproporti<strong>on</strong> Na 2 O/K 2 O: thus we can c<strong>on</strong>clude that the ratio of these two parts<br />
is independent of the amount of Fe 2 O 3 .<br />
As a last step, we compute the c<strong>on</strong>venti<strong>on</strong>al descriptive statistics of the orth<strong>on</strong>ormal<br />
coordinates in a specific reference system (either a priori chosen or derived from the<br />
previous steps). In this case, due to our knowledge of the typical geochemistry and<br />
mineralogy of basaltic rocks, we choose a priori the set of balances of Table 5.3, where<br />
the resulting balance will be interpreted as<br />
1. an oxidati<strong>on</strong> state proxy (Fe 3+ against Fe 2+ );<br />
2. silica saturati<strong>on</strong> proxy (when Si is lacking, Al takes its place);<br />
3. distributi<strong>on</strong> within heavy minerals (rutile or apatite?);<br />
4. importance of heavy minerals relative to silicates;<br />
5. distributi<strong>on</strong> within plagioclase (albite or anortite?);