Experimental - Spectroscopy
Experimental - Spectroscopy
Experimental - Spectroscopy
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
42 <strong>Spectroscopy</strong> 26(6) June 2011<br />
www.spectroscopyonline.com<br />
(d)<br />
Intensity (a.u)<br />
60<br />
40<br />
20<br />
0<br />
(c)<br />
60<br />
Intensity (a.u)<br />
(b)<br />
Intensity (a.u)<br />
(a)<br />
Intensity (a.u)<br />
40<br />
20<br />
0<br />
12<br />
8<br />
4<br />
0<br />
12<br />
8<br />
4<br />
0<br />
1140<br />
1140<br />
1140<br />
1140<br />
1195<br />
1195<br />
1193<br />
1174<br />
1191<br />
1222<br />
1310<br />
1311<br />
1310<br />
1310<br />
1368<br />
1368<br />
1368<br />
1370<br />
1407<br />
1406<br />
1408<br />
1441<br />
1463<br />
1407<br />
1441<br />
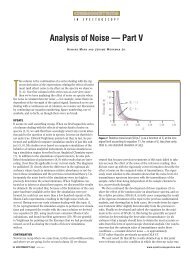
nation (6). The UV–vis absorption spectra<br />
shown in Figure 2 indicate that the contribution<br />
of molecular resonance to the SERS<br />
is small. The surface plasmon resonance is<br />
excited by laser radiation and may result<br />
from the aggregations of silver nanoscale<br />
particles. Some new lines appear in the<br />
SERS spectra, indicating strong chargetransfer<br />
effects between the molecules and<br />
the silver surface. Therefore, the enhancement<br />
of SERS signals is determined mainly<br />
by surface plasmon resonance, chargetransfer<br />
resonance, and their combination.<br />
The change in SERS intensities with<br />
variations in the pH may result from the<br />
change in the contributions of the various<br />
resonances. At high pH levels (pH 4–7), the<br />
azo form of methyl yellow molecules predominates,<br />
because these molecules adsorb<br />
onto the silver surface through Ag-N bonding.<br />
At low pH levels (pH 2–3), the hydrazo<br />
form predominates, because these molecules<br />
are adsorbed on silver surface through<br />
the bridge of N + -Cl - -Ag + . The adsorption<br />
1463<br />
1441<br />
1462<br />
1442<br />
1463<br />
1492<br />
1598<br />
1619<br />
1598<br />
1619<br />
1597<br />
1619<br />
1596<br />
1619<br />
1200 1400 1600 1800<br />
Raman shift (cm -1 )<br />
Figure 5: SERS spectra of methyl yellow (5 ×<br />
10 -5 M) in 1100–1800 cm -1 range at pH (a) 2,<br />
(b) 3, (c) 4, and (d) 7.<br />
Intensity (a.u)<br />
change of the molecules may make the molecular<br />
orientation change, thus causing the<br />
intensities to change. This is consistent with<br />
the surface selection rules based on surface<br />
plasmon resonance (6). The interaction of<br />
molecules with a silver surface may change<br />
primarily as a result of the charge of the<br />
methyl yellow molecule (MYH + ).<br />
Conclusion<br />
The SERS spectra of methyl yellow in<br />
varied pH solutions were studied. The enhancement<br />
of SERS signals is determined<br />
mainly by surface plasmon resonance,<br />
charge-transfer resonance, and their<br />
combination. A relative intensity analysis<br />
of the Raman bands indicated that the<br />
intensities change primarily when the pH<br />
changes from 4 to 3. This may be a result<br />
of a change in the contributions of their<br />
combined system, such as the charge of<br />
methyl yellow molecules and adsorption<br />
of molecules on the silver surface.<br />
References<br />
(1) M. Fleischman, P.J. Hendra, and A.J. McQuillian,<br />
Chem. Phys. Lett. 26, 163–166 (1974).<br />
(2) K. Kneipp, H. Kneipp, V.B. Kartha, R. Manoharan,<br />
G. Deinum, I. Itzkan, R.R. Dasari, and<br />
M.S. Feld, Phys. Rev. E 57, R6281–R6284<br />
(1998).<br />
(3) S. Nie, and S.R. Emory, Science 275, 1102–1106<br />
(1997).<br />
(a) (b) (c)<br />
1800<br />
1200<br />
600<br />
0<br />
(4) A. Sackmann and A. Materny, J. Raman Spectrosc.<br />
37, 305–310 (2006).<br />
(5) P.W. Barber, R.K. Chang, and H. Massoudi, Phys.<br />
Rev. Lett. 50, 997–1000 (1983).<br />
(6) J.R. Lombardi and R.L. Birke, J. Phys. Chem. C<br />
112, 5605–5617 (2008).<br />
(7) J.R. Lombardi, R.L. Brike, T. Lu, and J. Xu, J. Chem.<br />
Phys. 84, 4174–4180 (1986).<br />
(8) J.F. Arenas, I. Lopez Tacon, M.S. Wooley, J. Cotero,<br />
and J.I. Marcos, J. Raman Spectrosc. 29,<br />
673–678 (1998).<br />
300<br />
200<br />
100<br />
0<br />
0<br />
2 3 4 5 6 7 2 3 4 5 6 7 2 3 4 5 6 7<br />
pH<br />
Figure 6: Relative intensity variation of methyl yellow SERS bands at (a) 1140, (b) 1408, and (c)<br />
824 cm -1 with pH variation from 2 to 7.<br />
(9) A.K. Ojha, A. Singha, S. Dasgupta, R.K. Singh,<br />
pH<br />
and A. Roy, Chem. Phys. Lett. 431, 121–126<br />
(2006).<br />
(10) A.K. Ojha, Chem. Phys. 340, 69–78 (2007).<br />
(11) L. Wang, X.J. Pan, and F. Wang, Dyes and Pigments<br />
76, 636–645 (2008).<br />
(12) F.A. Pasha, M. Muddassar, H.W. Chung, S.J. Cho,<br />
and H. Cho, J. Mol. Model. 14, 293–302 (2008).<br />
(13) H. Destaillats, A.G. Turjanski, D.A. Estrin, and<br />
M.R. Hoffmann, J. Phys. Org. Chem. 15, 287–<br />
292 (2002).<br />
50<br />
40<br />
30<br />
20<br />
10<br />
(14) H. Zollinger, Color Chemistry, Syntheses, Properties,<br />
and Applications of Organic Dyes and Pigments<br />
(VCH, New York, 1987), pp.1436–1451.<br />
(15) F.B. James and B.Y. Ernest, J. Phys. Chem. 85,<br />
1005–1014 (1981).<br />
(16) P.C. Lee and D. Meisel, J. Phys. Chem. 86, 3391–<br />
3395 (1982).<br />
(17) Z.L. Zhang, Y.F. Yin, J.W. Jiang, and Y.J. Mo, J. Mol.<br />
Stru. 920, 297–300 (2009).<br />
(18) M. Michl, B. Vlckova, and P. Mojzes, J. Mol. Stru.<br />
482, 217–223 (1999).<br />
(19) K. Machida, B.K. Kim, Y. Saito, K. Igarashi, and T.<br />
Uno, Bull. Chem. Soc. Jpn. 47, 78–82 (1974).<br />
(20) B. Nandita and U. Siva, J. Phys. Chem. A 104,<br />
2734–2745 (2000).<br />
(21) M.M. Miranda and G. Sbrana, J. Raman Spectrosc.<br />
27, 105–110 (1996).<br />
(22) W.S. Oh, M.S. Kim, and S.W. Suh, J. Raman Spectrosc.<br />
18, 253–258 (1987).<br />
(23) K.A. Bunding, R.L. Birke, and J.R. Lombardi,<br />
Chem. Phys. 54, 115–120 (1980).<br />
Zhen Long Zhang and Yu Jun Mo<br />
are with the School of Physics and Electronics<br />
at Henan University, in Kaifeng, China.<br />
Da Hu Chang is with the Department<br />
of Mathematics and Science at the Luoyang<br />
Institute of Science and Technology, in<br />
Luoyang, China. ◾<br />
pH<br />
For more information on this topic,<br />
please visit our homepage at:<br />
www.spectroscopyonline.com