Hematopathology Resident / Fellow Manual - Department of ...
Hematopathology Resident / Fellow Manual - Department of ...
Hematopathology Resident / Fellow Manual - Department of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
DIVISION OF HEMATOPATHOLOGY<br />
HEMATOPATHOLOGY HANDBOOK<br />
FOR<br />
RESIDENTS AND FELLOWS<br />
October 2013<br />
STEVEN H. SWERDLOW, MD<br />
DIRECTOR, DIVISION OF HEMATOPATHOLOGY<br />
GRANT BULLOCK, MD<br />
LYDIA CONTIS, MD<br />
FIONA CRAIG, MD<br />
MIROSLAV DJOKIC, MD<br />
RAYMOND FELGAR, MD/PhD<br />
SARAH GIBSON, MD<br />
LISA ROBINSON, MD<br />
CHRISTINE ROTH, MD<br />
The Division acknowledges the help <strong>of</strong> Ms. L. Koerbel and Ms. J.<br />
Klimkowicz in putting this handbook together and keeping it up to date.
TABLE OF CONTENTS<br />
(Ctrl +click on blue hyperlink to go to section)<br />
IMPORTANT TELEPHONE NUMBERS LIST 5<br />
GENERAL OUTLINE FOR HEMATOPATHOLOGY ROTATION 7<br />
HEMATOPATHOLOGY PROGRESSIVE GOALS AND OBJECTIVES 11<br />
RESIDENT EVALUATION METHODS AND DOCUMENTATION 18<br />
CONFERENCE SCHEDULE AND RESPONSIBILITIES 19<br />
PEDIATRIC AND GENERAL HEMATOLOGY LABORATORY EXPERIENCE 26<br />
GENERAL/SPECIAL HEMATOLOGY LABORATORY EXPERIENCE CHECKLIST 29<br />
PEDIATRIC HEMATOPATHOLOGY CHECKLIST 41<br />
UPMC PRESBYTERIAN SHADYSIDE AUTOMATED TESTING LABORATORY 49<br />
PATHOLOGIST REVIEW FORM/CHP ATL PATHOLOGIST REVIEW FORM<br />
CLINICAL EXPERIENCE IN HEMATOLOGY 51<br />
CLINICAL HEMATOLOGY/PERFORMANCE OF BONE MARROW EXPERIENCE 52<br />
PERFORMANCE OF BONE MARROW ASPIRATIONS & BIOPSIES 53<br />
HEMATOPATHOLOGY ROTATION PERFORMANCE OF MARROW BIOPSIES AND ASPIRATES FORM 55<br />
FLOW CYTOMETRY LABORATORY EXPERIENCE 56<br />
FLOW CYTOMETRY ROTATION CHECKLIST 58<br />
FLOW CYTOMETRY PANELS, AVAILABLE ANTIBODIES, GUIDELINES, 60<br />
TEST SPECIFICATIONS FOR CLINICAL FLOW CYTOMETRY LABORATORY 71<br />
ICD9 CODES FOR FLOW 73<br />
ADULT BONE MARROW EXPERIENCE 75<br />
GENERAL TRAINEE RESPONSIBILITIES DURING BONE MARROW ROTATION 76<br />
SPECIFIC GUIDELINES FOR RESIDENTS AND FELLOWS ON BONE MARROW SERVICE 78<br />
BONE MARROW ADEQUACY CRITERIA 79<br />
POLICY FOR REVIEW OF BONE MARROW ASPIRATES AND BIOPSIES 80<br />
FORMS AND TEMPLATES FOR BONE MARROW SERVICE 82<br />
RECOMMENDATIONS FOR CONSISTENT TERMINOLOGY 93<br />
AJCC PROTOCOL FOR EXAMINATIOM OF SPECIMINS FROM PATIENTS WITH<br />
HEMATOPOIETIC NEOPLASMS INVOLVING THE BONE MARROW 96<br />
BONE MARROW LABORATORY TEST SPECIFICATIONS 109<br />
SUMMARY OF TEST SPECIFICATION FOR ANCILLARY LABORATORIES 111<br />
BONE MARROW AFTER HOURS PROCEDURES 113
TABLE OF CONTENTS (cont.)<br />
LYMPH NODE EXPERIENCE 117<br />
SURGICAL PATHOLOGY MANUAL: LYMPH NODE PROTOCOL 118<br />
SURGICAL PATHOLOGY MANUAL: SPLEEN PROTOCOL 127<br />
LYMPH NODE GROSS DICTATION-TEMPLATE 128<br />
POLICY FOR SOLID TISSUE SPECIMENS 129<br />
INSTRUCTIONS FOR FRESH TISSUE BIOPSIES RECEIVED FROM OUTSIDE INSTITUTIONS 130<br />
FRESH CASE LYMPH NODES AND OTHER SOLID TISSUES SENT FOR FLOW CYTOMETRY 131<br />
HELPFUL HINTS FOR HANDLING CONSULT BLOCKS &<br />
SENDING OUTSIDE BLOCKS TO HISTOLOGY 132<br />
RULES FOR HISTOLOGY 133<br />
GENERAL FORMATTING REQUIREMENTS 136<br />
LYMPH NODE/SOLID TISSUE ORGANIZATION OF SIGNOUT MATERIAL 142<br />
HEMATOPATHOLOGY CASE WORKSHEET 144<br />
LYMPH NODE ROTATION CHECKLIST 149<br />
AJCC PROTOCOL FOR EXAMINTION OF SPECIMENS FROM PATIENT WITH<br />
NON-HODGKIN LYMPHOMA/LYMPHOID NEOPLASMS 158<br />
AJCC PROTOCOL FOR THE EXAMINATION OF SPECIMENS FROM PATIENTS WITH<br />
HODGKIN LYMPHOMA 174<br />
IMMUNOHISTOCHEMISTRY 179<br />
IMMUNOSTAINS/IN-SITU HYBRIDIZATION LABORATORY AND ORDERING INFORMATION 180<br />
STAINS FREQUENTLY USED IN HEMATOPATHOLOGY: CODES AND REACTIVITIES 182<br />
COMPLETE IMMUNOHISTOCHEMICAL STAIN ABBREVIATIONS/CODES 185<br />
IN-SITU HYBRIDIZATION PROBES AVAILABLE 193<br />
IHC/ISH REPORTING TEMPLATES 194<br />
MOLECULAR DIAGNOSTIC AND CYTOGENETICS 197<br />
FLUORESCENCE IN SITU HYBRIDIZATION – PITTSBURGH<br />
CYTOGENETICS LABORATORY 199<br />
COPATH AND DICTATION POINTERS 208<br />
DICTATING & PROOFING TISSUE REPORTS 210<br />
SYNOPTICS 213<br />
WEB-BASED RESOURCES 230
Contents <strong>of</strong> Supplementary Information<br />
See the following hard copy supplementary information:<br />
1. Schedules<br />
• Master <strong>Hematopathology</strong><br />
• Flow Cytometry Conference<br />
• Journal Club<br />
• Patient Safety and Risk Management <strong>Hematopathology</strong> Conference<br />
• Pediatric Tumor Board<br />
• Conference Schedule<br />
• <strong>Hematopathology</strong> Core <strong>Resident</strong> Rotation<br />
2. Forms to turn into <strong>Hematopathology</strong> Coordinator Linda Koerbel:<br />
• Performance <strong>of</strong> Marrow Biopsies and Aspirates Form (2)<br />
• Laboratory Hematology Check List<br />
• Flow Cytometry Experience Checklist<br />
• Lymph Node Experience Checklist<br />
• Pediatric Hematology Experience Checklist<br />
• Bone Marrow Experience Checklist<br />
3. Material for Laboratory Hematology<br />
• Red Cell Morphology Classification<br />
• Complete Blood Count Evaluation<br />
• Continuing Education in Clinical Chemistry and Hematology Conference Responsibilities<br />
4. Miscellaneous<br />
• Progressive Goals and Objectives<br />
• Faculty/<strong>Fellow</strong> Phone List<br />
• Sure-handed Sampling/Easing the Trauma <strong>of</strong> Bone Marrow Collection<br />
• <strong>Resident</strong> Evaluation Methods<br />
• Dictating and Pro<strong>of</strong>ing Tissue Reports<br />
• CD<br />
• <strong>Hematopathology</strong> Handbook for <strong>Resident</strong>s and <strong>Fellow</strong>s<br />
• Automated Hematology Instrumentation<br />
• Grossing Fresh Lymph Nodes (PowerPoint Presentation)<br />
• Neutrophil Oxidative Burst Assay (NOBA)<br />
• Hemoglobinopathies (PowerPoint Presentation)<br />
• Chromatin interpretation guide for hemoglobinopathies (PDF)
HEMATOPATHOLOGY<br />
FACULTY<br />
Long Range<br />
Pager<br />
In House<br />
Pager<br />
Office #<br />
Coordinator/Secretary<br />
Grant Bullock, MD 412-958-6254 10356 412-624-7523 Jessica Klimkowicz 412-647-5191<br />
Lydia Contis, MD 412-433-9364 2296 412-647-0264 Jessica Klimkowicz 412-647-5191<br />
Fiona Craig, MD 412-958-2896 2462 412-647-8504 Jessica Klimkowicz 412-647-5191<br />
Miroslav Djokic, MD 412-958-5267 14609 412-692-2128 Jessica Klimkowicz 412-647-5191<br />
Raymond Felgar, MD,<br />
PhD<br />
412-958-6088 2825 412-647-8780 Jessica Klimkowicz 412-647-5191<br />
Sarah Gibson, MD 412-958-5723 13155 412-647-4162 Jessica Klimkowicz 412-647-5191<br />
Lisa Robinson, MD 412-958-8449 10586 412-647-0365 Jessica Klimkowicz 412-647-5191<br />
Christine Roth, MD 412-958-4985 2282 412-692-2090 Jessica Klimkowicz 412-647-5191<br />
Steven H. Swerdlow, MD 412-392-7445 2826 412-647-5191 Linda Koerbel 412-578-9423<br />
FELLOWS<br />
Long Range In House<br />
Pager<br />
Pager<br />
Office #<br />
Coordinator/Secretary<br />
Christopher Cogbill, MD 412-958-7419 8338 412-647-1877 N/A<br />
Bevan Tandon, MD 412-958-7421 8339 412-647-0435 N/A<br />
Charlene Hellman, MD 412-958-7434 8359 412-578-9239 N/A<br />
Lymph Node Assistant<br />
Lisa Fitchwell 412-958-7432 10768 412-647-5869 N/A<br />
Michelle Asturi --------------- --------------- 412-647-0263 N/A<br />
Cytogenetics/Hours <strong>of</strong> 412-641-6688 (Weekend pager: 412-917-9458-messages will be checked until 3PM)<br />
FISH Lab 412-641-3434<br />
Dr. Urvashi Surti 412-917-9333 --------------- 412-641-4267<br />
Dr. Susanne Gollin --------------- --------------- 412-624-5390 Noel Eisel Harrie<br />
Maureen Sherer --------------- --------------- 412-641-6685 ---------------<br />
Technologist Pager<br />
412-917-9458 (Sat./Sun./Holidays until 3:00pm)<br />
Molecular Diagnostics 412-864-6150 (CLB)<br />
Molec <strong>Fellow</strong>s 412-864-6155<br />
Molec Sign-Out 412-864-6153<br />
Dr. Zoltan Oltvai --------------- --------------- 412-864-6150<br />
Dr. Tim Oury --------------- --------------- 412-648-9659<br />
Dr. Marie DeFrances --------------- --------------- 412-648-8346<br />
Miscellaneous Phone Number Location Supervisor Lead Technologist<br />
Bone Marrow Lab 412-647-0263 G325.1 Celina Fortunato Celina Fortunato 412-802-3272<br />
Bone Marrow Audix 412-802-3273 “Non-Stat” Requests<br />
Bone Marrow Sign Out 412-647-5881 G315 --------------- ------------------<br />
Histology 412-647-7660 CLB<br />
Marina Rahman<br />
412-864-6123<br />
Tisha<br />
Harrison<br />
5am-1:30pm<br />
immunopero<br />
xidase<br />
issues<br />
IPEX 412-647-7663<br />
Lymph Node Sign Out /<br />
Resource Room<br />
412-647-5262 G323 --------------- ------------------<br />
Mike<br />
Swiatkowski<br />
7-3:30<br />
Routine<br />
histology and<br />
special stain<br />
issues<br />
Consult Accessioning 412-864-6175 CLB 9032 Celina Fortunato Celina Fortunato 412-802-3272<br />
Automated Testing Lab<br />
412-647-1022/<br />
heme bench 76199<br />
5 th Fl CLB<br />
SHY Automated Testing<br />
Lab<br />
412-623-1595 WG02.18<br />
Flow Laboratory 412-864-6173<br />
Flow Laboratory Extras 412-864-6182<br />
CLB 9032<br />
Katie Mulvey<br />
412-647-5662<br />
Michael Kaib<br />
412-623-2322<br />
Pager<br />
412-565-9553<br />
Betty Austin 77864 (Heme)<br />
Karen Freilino 412-864-6180<br />
Shadyside Fl<br />
SHY Hematology Lab 412-623-6011<br />
Darla Lower Tammy Garrett<br />
G-Main 412-623-1595<br />
Microlab Adult 412-647-3727 PUH A802 Frances Hardic 412-647-7711<br />
412-692-5325 CHP<br />
Lorraine Heffelfinger 412-692-7611 /<br />
CHP ATL<br />
412-692-5665 Lawrenceville Marianne Riazzi 412-692-9836<br />
Robyn<br />
Darwick 3-<br />
11:30<br />
Evening<br />
routine &<br />
immunopero<br />
xidase issues
GENERAL OUTLINE FOR<br />
HEMATOPATHOLOGY<br />
ROTATION
General Outline for <strong>Hematopathology</strong> Rotations<br />
Core Rotation for <strong>Resident</strong>s<br />
The approximately three-month core hematopathology rotation <strong>of</strong>fers the resident an<br />
introduction to the many facets <strong>of</strong> this complex field. It is hoped that the resident will begin to<br />
become familiar with the multiparameter approach to adult and pediatric diagnostic<br />
hematopathology (bone marrows and lymph nodes) as well as with techniques used in general<br />
and special hematology laboratories, and the flow cytometry laboratory. Finally he/she will learn<br />
about major neoplastic and non-neoplastic disease entities that involve the hematopoietic and<br />
lymphoid cell lineage. If interested, more advanced subsequent rotations can be arranged in<br />
one or more areas within the division. It is fully recognized that the resident cannot fully achieve<br />
all <strong>of</strong> the objectives listed within a period <strong>of</strong> three months. The different sections within the<br />
rotation are usually, but not always carried out in the order they are listed here. The educational<br />
resources noted below are located in rooms G304 (conference room), G315 (bone marrow signout<br />
room) and G323 (lymph node sign-out room) depending on their subject matter.<br />
Cytogenetics is a separate rotation, but residents will learn how cytogenetic data is used in<br />
diagnostic hematopathology.<br />
Syllabus Statement Concerning Students with Disabilities<br />
If you have a disability for which you are or may be requesting an accommodation, you are<br />
encouraged to contact Dr. Swerdlow, Dr. Roth or their designate prior to your rotation so<br />
reasonable accommodations can be made.<br />
Elective Rotations<br />
This handbook also serves as a resource for upper level resident rotations that can concentrate<br />
on any <strong>of</strong> the areas in hematopathology.<br />
<strong>Fellow</strong> Rotations<br />
This handbook also is a major supplement to the fellow handbook as they also follow the basic<br />
procedures outlined here for the rotations that overlap with hematopathology resident rotations.<br />
The expectations are greater for the fellows in terms <strong>of</strong> their knowledge base, clinical skills and<br />
ability to utilize multiple resources to make specific diagnoses. Accordingly they are given<br />
enhanced responsibilities.<br />
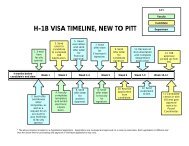
<strong>Hematopathology</strong> Twelve Week Core <strong>Resident</strong> Rotation<br />
Week<br />
Activities<br />
1 Orientation with Dr. Roth or designee.<br />
Lymph Node<br />
2 Lymph Node<br />
3 Lymph Node<br />
4 Lymph Node<br />
5 Peds/Wet & ASCP image set/BM tech review<br />
6 Peds/Wet<br />
7 Peds/Wet<br />
8 Days 1-3 AM Shadyside (Go to Hemepath Conference Wed AM)<br />
Days 3 PM – 4,5 Flow Rotation<br />
9 Adult Bone Marrow<br />
10 Adult Bone Marrow<br />
11 Adult Bone Marrow<br />
12 Adult Bone Marrow
1. Lymph Node Pathology (~ 4 weeks)<br />
1. Lymph Node Sign Out.<br />
2. Review <strong>of</strong> educational materials (See Lymph Node Experience section).<br />
Goals and Objectives:<br />
1. Learn the use <strong>of</strong> multiparameter approach to diagnostic lymph node pathology as<br />
well as extranodal hematopoietic/lymphoid proliferations.<br />
2. Learn normal nodal histology and basic reactive patterns.<br />
3. Begin to develop a basic understanding <strong>of</strong> Hodgkin’s Lymphoma, the non-<br />
Hodgkin’s lymphomas and reactive lymphoid hyperplasias. Be able to diagnose<br />
straightforward cases <strong>of</strong> the above.<br />
4. Complete lymph node rotation checklist.<br />
2. Pediatric <strong>Hematopathology</strong> and General/Special Hematology Laboratory<br />
Experience (~ 3 weeks)<br />
Trainees should report to the pathologist signing out CHP bone marrows and to Dr. Contis or<br />
her designate after their general rotation orientation or at the start <strong>of</strong> the rotation. Trainees<br />
should also meet with Dr. Contis or her designate at the midpoint to review progress on the<br />
rotation and at the completion <strong>of</strong> the rotation. Inform the pathologist covering the general<br />
hematology laboratory as well. The trainee will give one ~15 minute presentation near the end<br />
<strong>of</strong> this section <strong>of</strong> the rotation. This period should also be used to review the ASCP image series<br />
on normal and abnormal peripheral blood and bone marrow examinations.<br />
Pediatric <strong>Hematopathology</strong> Experience<br />
1. Introduction to basic bone marrow/peripheral blood interpretation.<br />
2. Pediatric Bone Marrow Sign out.<br />
3. Observation <strong>of</strong> pediatric marrow examination procedures.<br />
4. Review pediatric material from Automated Testing Laboratory and Flow<br />
Cytometry Laboratory.<br />
5. Review <strong>of</strong> educational materials (See Pediatric <strong>Hematopathology</strong> Experience<br />
section).<br />
Goals and Objectives:<br />
1. Develop basic skills in the interpretation <strong>of</strong> peripheral blood, bone marrow and<br />
fluid evaluations.<br />
2. Develop familiarity with issues unique to pediatric hematopathology, both<br />
pathologic and clinical.<br />
3. Develop basic skills in hematopathologic ancillary studies.<br />
4. Complete pediatric hematopathology checklist.<br />
5. Complete pediatric bone marrow observation form.
General/Special Hematology Laboratory Experience<br />
1. Automated Testing Laboratory Experience.<br />
2. Special Hematology Testing Experience.<br />
3. Review <strong>of</strong> educational materials.<br />
4. Presentation to technologists.<br />
Goals and Objectives:<br />
1. Learn the major aspects <strong>of</strong> non-neoplastic hematology including red blood cell,<br />
white blood and platelet abnormalities and the way in which the laboratory helps<br />
in the diagnosis and follow-up <strong>of</strong> hematologic/lymphoid neoplasms.<br />
2. Understand the principles <strong>of</strong> urinalysis and how it is used to help diagnose renal<br />
and systemic disorders.<br />
3. Understand automated hematology and urinalysis instrumentation.<br />
4. Develop understanding <strong>of</strong> how a large complex patient-oriented clinical<br />
laboratory facility is managed. This includes an appreciation <strong>of</strong> the scope <strong>of</strong> the<br />
testing, testing methodology and documentation <strong>of</strong> test accuracy.<br />
5. Learn the scope and practices <strong>of</strong> “Special Hematology” testing and how it is<br />
utilized to diagnose hematologic disorders.<br />
6. Complete general/special hematology experience checklist.<br />
3. Clinical experience in Hematology/Performance <strong>of</strong> Bone Marrows (with<br />
Hematology/Oncology Division) (2 ½ days)<br />
Attend varied clinics at UPMC-Shadyside/Hillman Cancer Center to observe the clinical<br />
aspects <strong>of</strong> hematology and understand the needs <strong>of</strong> both patients and their physicians.<br />
Trainees are expected to learn how to perform bone marrow aspirations and biopsies.<br />
They should aim to observe and then perform several marrows. Because it is expected<br />
that during this rotation residents will perform no more that 5 marrows, they should<br />
complete this requirement on their later VA rotation (Dr. M. Melhem). Be sure to<br />
complete the BM performance form that requires signatures <strong>of</strong> the person who<br />
supervised your marrow examinations and the pathologist who reviewed the slides.<br />
NOTE: Appropriate dress is required to see patients (white coat, men need to wear a<br />
tie).<br />
Goals and Objectives:<br />
1. Gain experience performing bone marrow aspirates and biopsies.<br />
2. Learn more about clinical implications <strong>of</strong> hematopathology diagnoses and impact<br />
on patients as a person.<br />
3. Provide opportunities to perform bone marrow examinations.<br />
4. Learn what type <strong>of</strong> consultations clinicians expect from hematopathologists.<br />
5. Complete the bone marrow performance form.
4. Flow Cytometry Laboratory Experience (2½ days)<br />
Understanding <strong>of</strong> flow cytometric immunophenotypic techniques and interpretation <strong>of</strong> the<br />
resultant data is an integral part <strong>of</strong> all the bone marrow and lymph node rotations. In<br />
addition, however there is a brief concentrated exposure to the flow cytometry laboratory<br />
including the technical aspects <strong>of</strong> flow cytometry and the basic operation <strong>of</strong> a flow<br />
cytometry laboratory. The resident should report to Karen Freilino or their designate.<br />
Goals and Objectives:<br />
1. Understand sample preparation; basic flow cytometry, quality control, gating on<br />
specific cell populations, and determination <strong>of</strong> positive versus negative staining<br />
and methods <strong>of</strong> data presentation.<br />
2. Know indications for testing, taking into account cost effective medicine<br />
3. Complete flow cytometry checklist.<br />
5. Adult Bone Marrow Experience (~ 4 weeks)<br />
A. Limited Sessions with Adult Bone Marrow Technologist.<br />
B. Responsibility for review <strong>of</strong> selected cases prior to sign-out.<br />
C. Participation in sign-out with staff.<br />
D. Review <strong>of</strong> educational materials (See Adult Bone Marrow Experience section).<br />
Goals and Objectives:<br />
1. Learn normal and abnormal blood cell morphology.<br />
2. Learn basic approach to bone marrow aspirate, biopsy and particle preparation<br />
interpretation.<br />
3. Begin to become familiar with the use <strong>of</strong> ancillary studies used in the diagnosis <strong>of</strong><br />
bone marrow examinations.<br />
4. Begin to develop a basic understanding <strong>of</strong> the more common neoplastic and nonneoplastic<br />
disorders which involve the marrow: acute and chronic leukemias,<br />
myelodysplasias, myeloproliferative disorders, anemias, thrombocytopenias,<br />
leukopenias, thrombocytosis, leukocytosis, infections (including HIV), and<br />
metastatic neoplasms. Be able to diagnose straightforward cases <strong>of</strong> the above.<br />
5. Complete bone marrow rotation checklist.
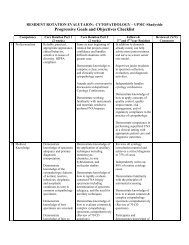
<strong>Hematopathology</strong> Progressive Goals and Objectives<br />
Competency<br />
Core Rotation - 1st to<br />
3 rd Year <strong>Resident</strong><br />
Beginning <strong>of</strong><br />
Rotation<br />
Core Rotation – 1 st<br />
to 3 rd year <strong>Resident</strong><br />
Later in Rotation<br />
Elective Rotation - 3rd and<br />
4th year <strong>Resident</strong><br />
<strong>Fellow</strong><br />
First Year<br />
<strong>Fellow</strong><br />
Second Year<br />
Post <strong>Fellow</strong>ship<br />
Experience<br />
Pr<strong>of</strong>essionalism Reliable, punctual,<br />
appropriate<br />
appearance, ethical<br />
behavior, sensitive to<br />
issues <strong>of</strong> diversity,<br />
HIPAA compliant<br />
Patient Care<br />
Same as near<br />
beginning <strong>of</strong> rotation<br />
but projects more<br />
confidence and<br />
handles difficult<br />
situations with<br />
greater ease.<br />
In addition to elements<br />
already noted, can help advise<br />
more junior trainees and serve<br />
as a more senior role model.<br />
Preview marrow<br />
aspirate smears with<br />
Preview marrow<br />
aspirate smears<br />
Write and dictate reports for<br />
most routine cases.<br />
direct faculty guidance. semi-independently,<br />
Review cases, record<br />
observations,<br />
formulate differential<br />
diagnosis.<br />
directly interact with<br />
technologists.<br />
Review cases, record<br />
observations,<br />
formulate more<br />
complete differential<br />
diagnosis<br />
In addition to elements<br />
noted for residents,<br />
functions so that others<br />
perceive fellow more like<br />
a junior faculty member.<br />
Create a pr<strong>of</strong>essional<br />
CV. Conduct a<br />
successful job search if<br />
not continuing as a<br />
fellow.<br />
Independently work-up<br />
and complete the<br />
majority <strong>of</strong> cases.<br />
In addition to prior<br />
accomplishments,<br />
interacts with other<br />
faculty and clinicians like<br />
a more confident junior<br />
faculty member, able to<br />
construct and maintain<br />
pr<strong>of</strong>essional c.v. and<br />
biosketch<br />
Able to provide a<br />
complete diagnostic<br />
report to attending<br />
faculty with minimal<br />
required changes.<br />
Formulate list <strong>of</strong><br />
immunohistochemical<br />
stains, cytochemical<br />
stains, flow antibody<br />
combinations to<br />
resolve differential<br />
diagnosis. Review data<br />
from ancillary studies<br />
Formulate more<br />
educated list <strong>of</strong><br />
immunohistochemical<br />
stains, cytochemical<br />
stains, flow antibody<br />
combinations to<br />
resolve differential<br />
diagnosis. Review<br />
Independently order ancillary<br />
studies in a resource<br />
conscious way on most<br />
routine cases.<br />
Independently order<br />
ancillary studies in a<br />
resource conscious way<br />
on routine and most<br />
complex cases.<br />
Independently order<br />
ancillary studies in a<br />
resource conscious way<br />
on virtually all cases.
Competency<br />
Core Rotation - 1st to<br />
3 rd Year <strong>Resident</strong><br />
Beginning <strong>of</strong><br />
Rotation<br />
Core Rotation – 1 st<br />
to 3 rd year <strong>Resident</strong><br />
Later in Rotation<br />
Elective Rotation - 3rd and<br />
4th year <strong>Resident</strong><br />
<strong>Fellow</strong><br />
First Year<br />
<strong>Fellow</strong><br />
Second Year<br />
Post <strong>Fellow</strong>ship<br />
Experience<br />
and record<br />
interpretation.<br />
data from ancillary<br />
studies and record<br />
more complete<br />
interpretation.<br />
Gross specimens for<br />
lymphoma work-up<br />
with directed<br />
supervision.<br />
With explicit directions,<br />
interact with clinicians<br />
and support staff.<br />
Be able to provide<br />
basic review <strong>of</strong><br />
peripheral blood and<br />
interpret most common<br />
hematology tests.<br />
Gross specimens for<br />
lymphoma work-up<br />
with supervision as<br />
needed (after<br />
consulting fellow or<br />
appropriate faculty).<br />
With less explicit<br />
directions, interact<br />
with clinicians and<br />
support staff.<br />
Be able to provide Provide<br />
basic review <strong>of</strong><br />
peripheral blood,<br />
fluids and urines and<br />
interpret most<br />
standard hematology<br />
tests.<br />
Gross specimens for<br />
lymphoma work-up with<br />
limited supervision and select<br />
ancillary testing independently<br />
for most routine cases.<br />
Function as a critical<br />
consultant to clinical<br />
physicians and support staff<br />
with some supervision.<br />
consultative/laboratory report<br />
for general and special<br />
hematology tests, peripheral<br />
blood and fluid reviews<br />
working with faculty on more<br />
complex cases and with<br />
limited assistance on less<br />
complex cases.<br />
Gross specimens for<br />
lymphoma work-up with<br />
very limited supervision<br />
and select ancillary<br />
testing independently for<br />
the majority <strong>of</strong> cases.<br />
Be able to help instruct<br />
junior trainees.<br />
Independently function<br />
as a critical consultant to<br />
clinical physicians.<br />
Provide<br />
consultative/laboratory<br />
report for general and<br />
special hematology<br />
tests, peripheral blood<br />
and fluid reviews on<br />
simple and complex<br />
cases relatively<br />
independently but with<br />
final approval by faculty<br />
member.<br />
Able to gross and triage<br />
specimens<br />
independently and to<br />
supervise and instruct<br />
more junior trainees.<br />
Able to supervise more<br />
junior trainee’s<br />
presentations and<br />
provide guidance for<br />
preparation.<br />
Provide<br />
consultative/laboratory<br />
report for general and<br />
special hematology<br />
tests, peripheral blood<br />
and fluid reviews in all<br />
cases with only limited<br />
supervision.
Competency<br />
Core Rotation - 1st to<br />
3 rd Year <strong>Resident</strong><br />
Beginning <strong>of</strong><br />
Rotation<br />
Core Rotation – 1 st<br />
to 3 rd year <strong>Resident</strong><br />
Later in Rotation<br />
Elective Rotation - 3rd and<br />
4th year <strong>Resident</strong><br />
<strong>Fellow</strong><br />
First Year<br />
<strong>Fellow</strong><br />
Second Year<br />
Post <strong>Fellow</strong>ship<br />
Experience<br />
Medical<br />
Knowledge<br />
Observe how others<br />
handle laboratory<br />
management issues.<br />
Present at interdepartmental<br />
CPC<br />
conferences with<br />
extensive supervision.<br />
Knowledge <strong>of</strong><br />
morphology and<br />
immunophenotype <strong>of</strong><br />
normal lymph node,<br />
spleen, bone marrow<br />
and peripheral blood.<br />
Knowledge <strong>of</strong><br />
multiparameter<br />
approach to diagnosis<br />
<strong>of</strong> hematologic<br />
disorders.<br />
Recognize some <strong>of</strong> the<br />
more common<br />
neoplastic and nonneoplastic<br />
disorders.<br />
Know basic<br />
immunophenotypic/<br />
genotypic/cytogenetic<br />
features where<br />
Participate with<br />
faculty/senior<br />
technical staff in<br />
laboratory<br />
management issues.<br />
Present at interdepartmental<br />
CPC<br />
Get directly involved in<br />
laboratory management<br />
issues with supervision.<br />
Present at inter-departmental<br />
CPC conferences with limited<br />
conferences with less supervision.<br />
direct supervision.<br />
Know criteria for Know criteria for some <strong>of</strong> the<br />
major neoplastic and less common<br />
non-neoplastic hematopathologic entities in<br />
hematopathologic addition to those for major<br />
entities.<br />
entities.<br />
Know specific<br />
approach used to<br />
diagnose major<br />
neoplastic and nonneoplastic<br />
hematologic entities.<br />
Recognize additional<br />
common neoplastic<br />
and non-neoplastic<br />
disorders and know<br />
ways in which<br />
specific entities are<br />
further subdivided. In<br />
addition to basic<br />
Recognize most common and<br />
some uncommon neoplastic<br />
and non-neoplastic disorders<br />
<strong>of</strong> the hematolymphoid system<br />
and know the<br />
immunophenotypic,<br />
cytogenetic and genotypic<br />
characteristics.<br />
Get involved in<br />
laboratory management<br />
issues with more limited<br />
supervision.<br />
Participate in continuing<br />
education <strong>of</strong><br />
technologists and<br />
support staff to improve<br />
patient care.<br />
Independently present at Presents cases at<br />
inter-departmental CPC clinical CPC<br />
conferences.<br />
conferences without<br />
supervision.<br />
Have an extensive<br />
knowledge <strong>of</strong> broad<br />
range <strong>of</strong> neoplastic and<br />
non-neoplastic<br />
hematopoietic/lymphoid<br />
disorders and other<br />
disorders that involve or<br />
affect the<br />
hematolymphoid system<br />
including the pathologic<br />
and clinical aspects <strong>of</strong><br />
these disorders.<br />
Further increase<br />
hematopathology<br />
knowledge base in terms<br />
<strong>of</strong> rare entities and<br />
variations within more<br />
common entities. Learn<br />
more about the type <strong>of</strong><br />
cases that lack a<br />
definitive diagnosis.<br />
Demonstrates ability to<br />
apply and discuss<br />
knowledge learned from<br />
instructional workshops<br />
or conferences attended.<br />
Recognizes broad range Demonstrates an<br />
<strong>of</strong> hematologic disorders appreciation <strong>of</strong> the<br />
and recognizes when a<br />
definitive diagnosis<br />
cannot be rendered or<br />
where consultative help<br />
may be required.<br />
limitation(s) <strong>of</strong> current<br />
diagnostic<br />
schemes/classification<br />
systems (i.e. shows<br />
recognition for “gray<br />
zones” in diagnosis).
Competency<br />
Core Rotation - 1st to<br />
3 rd Year <strong>Resident</strong><br />
Beginning <strong>of</strong><br />
Rotation<br />
Core Rotation – 1 st<br />
to 3 rd year <strong>Resident</strong><br />
Later in Rotation<br />
Elective Rotation - 3rd and<br />
4th year <strong>Resident</strong><br />
<strong>Fellow</strong><br />
First Year<br />
<strong>Fellow</strong><br />
Second Year<br />
Post <strong>Fellow</strong>ship<br />
Experience<br />
appropriate.<br />
Know basic<br />
components <strong>of</strong><br />
complete blood count<br />
and how they are<br />
obtained.<br />
Practice-based Become familiar with<br />
Learning<br />
basic hematopathology<br />
educational resources.<br />
ancillary data<br />
features, know<br />
pathophysiologic<br />
features <strong>of</strong> major<br />
entities. Complete<br />
greater than 80% <strong>of</strong><br />
resident version <strong>of</strong><br />
rotation checklist.<br />
Know basic<br />
components <strong>of</strong><br />
complete blood count<br />
and other major<br />
hematology tests and<br />
how they are<br />
obtained including<br />
major pitfalls. Also<br />
know basic principles<br />
<strong>of</strong> fluid and urinalysis<br />
interpretations.<br />
Know disease<br />
entities where<br />
diagnosis is based in<br />
large part on<br />
hematology<br />
laboratory testing.<br />
Completes more <strong>of</strong><br />
appropriate checklists and<br />
sees more entities previously<br />
encountered through reading.<br />
Complete greater than 90% <strong>of</strong><br />
resident version <strong>of</strong> rotation<br />
checklist.<br />
Know full armamentarium <strong>of</strong><br />
hematology testing, the<br />
purpose <strong>of</strong> each test and how<br />
to interpret combinations <strong>of</strong><br />
tests. Know new<br />
developments in hematology<br />
instrumentation.<br />
Search literature for Critically analyze literature<br />
information pertaining and other sources <strong>of</strong> new<br />
to cases and apply it information pertaining to<br />
to diagnostic cases.<br />
appraisals at sign-out<br />
Complete “extended<br />
version” <strong>of</strong> all rotation<br />
checklists.<br />
In addition to resident<br />
accomplishments, know<br />
details <strong>of</strong> more esoteric<br />
testing and what is on<br />
the horizon for<br />
laboratory hematology.<br />
Know how to evaluate<br />
new instrumentation.<br />
Be able to teach others<br />
about laboratory<br />
hematology including<br />
factual and interpretive<br />
elements.<br />
Have a broad<br />
Master all skill<br />
knowledge <strong>of</strong> the expectations listed for<br />
hematopathology more junior residents<br />
resources and literature and first year fellow.<br />
and be able to apply this Use information
Competency<br />
Core Rotation - 1st to<br />
3 rd Year <strong>Resident</strong><br />
Beginning <strong>of</strong><br />
Rotation<br />
Core Rotation – 1 st<br />
to 3 rd year <strong>Resident</strong><br />
Later in Rotation<br />
Elective Rotation - 3rd and<br />
4th year <strong>Resident</strong><br />
<strong>Fellow</strong><br />
First Year<br />
<strong>Fellow</strong><br />
Second Year<br />
Post <strong>Fellow</strong>ship<br />
Experience<br />
Start to develop<br />
diagnostic differentials<br />
for some <strong>of</strong> the more<br />
common neoplastic<br />
and non-neoplastic<br />
disorders with<br />
significant faculty input.<br />
and at conferences.<br />
Knows the differential<br />
diagnoses to<br />
consider for more<br />
commonly<br />
encountered<br />
neoplastic and nonneoplastic<br />
disorders.<br />
Able to construct more<br />
extensive differentials and<br />
apply knowledge by deciding<br />
what stains and ancillary<br />
testing would aid in<br />
distinguishing amongst the<br />
diagnostic possibilities being<br />
considered.<br />
information to daily<br />
practice including<br />
dealing with unusual<br />
cases<br />
Demonstrates ability to<br />
use textbooks and<br />
medical literature to<br />
construct a differential<br />
diagnosis for most cases<br />
and decide what<br />
ancillary testing would<br />
be useful.<br />
independently to alter<br />
personal practice.<br />
Demonstrates ability to<br />
apply knowledge from<br />
medical literature in<br />
constructing a diagnostic<br />
differential or choosing<br />
an appropriate work-up<br />
strategy <strong>of</strong> stains,<br />
ancillary testing, etc.<br />
Construct reports<br />
based on others’<br />
examples.<br />
Interpersonal/ Present with clarity in<br />
Communication conference settings<br />
Skills with significant faculty<br />
Improve reports<br />
based on comments<br />
received back from<br />
the faculty.<br />
Present with clarity in<br />
conference settings<br />
with minimal faculty<br />
Produce reports based on<br />
comments received back from<br />
the faculty who require few, if<br />
any, changes.<br />
Develop complete<br />
reports that reflect<br />
divisional style based on<br />
continued input from<br />
faculty, integrating the<br />
best suggestions from<br />
varied individuals.<br />
Independently use other<br />
colleagues and faculty<br />
as learning resources.<br />
Have established style<br />
for producing final<br />
reports that reflects an<br />
integration <strong>of</strong> input from<br />
varied faculty and<br />
integrate additional<br />
suggestions received on<br />
reports.<br />
Demonstrates ability to<br />
utilize other pr<strong>of</strong>essional<br />
colleagues as learning<br />
resource(s).
Competency<br />
Core Rotation - 1st to<br />
3 rd Year <strong>Resident</strong><br />
Beginning <strong>of</strong><br />
Rotation<br />
Core Rotation – 1 st<br />
to 3 rd year <strong>Resident</strong><br />
Later in Rotation<br />
Elective Rotation - 3rd and<br />
4th year <strong>Resident</strong><br />
<strong>Fellow</strong><br />
First Year<br />
<strong>Fellow</strong><br />
Second Year<br />
Post <strong>Fellow</strong>ship<br />
Experience<br />
guidance.<br />
assistance.<br />
System based<br />
Practice<br />
Works well with<br />
technologists and<br />
support staff and<br />
learns from them.<br />
Contact clinicians to<br />
obtain clinical and<br />
other information.<br />
Know and utilize basic<br />
aspects <strong>of</strong> resources<br />
available in health<br />
system i.e. computer<br />
systems (CoPath,<br />
MARS), laboratories<br />
(hematology,<br />
molecular diagnostics,<br />
cytogenetics,<br />
histology), grossing,<br />
bone marrow<br />
laboratories.<br />
Greater interaction<br />
with technologists,<br />
including<br />
demonstrating an<br />
ability to teach them.<br />
Able to convey<br />
straightforward<br />
information to<br />
clinicians.<br />
Know and more fully<br />
utilize resources<br />
available in health<br />
system.<br />
Can serve as a greater<br />
resource for technical staff.<br />
Discuss preliminary reports<br />
and diagnoses with clinicians<br />
with ease. Able to convey<br />
more complex information to<br />
clinicians and consulting<br />
pathologists.<br />
Learn about outside regulatory Learn about the<br />
agencies/organizations. administrative and<br />
Develop an appreciation <strong>of</strong> technical functions <strong>of</strong><br />
basic healthcare/pathology running the Division <strong>of</strong><br />
related financial issues. <strong>Hematopathology</strong>.<br />
Perform a mock CAP Perform a mock CAP<br />
inspection, if possible. inspection, if possible.<br />
Demonstrates the ability Able to educate<br />
to present information to technologists and<br />
technologists and junior residents with ease in<br />
residents at levels more impromptu settings<br />
appropriate for the as appropriate.<br />
audience.<br />
Proactively seeks<br />
opportunities to educate<br />
others.<br />
Able to convey complex Able to function as a<br />
information to clinicians junior faculty in terms <strong>of</strong><br />
and consulting providing consultative<br />
pathologists and can information to staff<br />
answer questions about pathologists at UPMC<br />
diagnoses or work-up. and elsewhere as well<br />
Also able to discuss as with clinicians.<br />
clinical implications <strong>of</strong><br />
diagnoses in depth.<br />
Demonstrates<br />
understanding <strong>of</strong> more<br />
complex personnel<br />
management issues.<br />
Understands the various<br />
components <strong>of</strong> a<br />
diagnostic<br />
hematopathology<br />
service and the<br />
interaction with other<br />
related, but separate<br />
services, such as
Competency<br />
Core Rotation - 1st to<br />
3 rd Year <strong>Resident</strong><br />
Beginning <strong>of</strong><br />
Rotation<br />
Core Rotation – 1 st<br />
to 3 rd year <strong>Resident</strong><br />
Later in Rotation<br />
Elective Rotation - 3rd and<br />
4th year <strong>Resident</strong><br />
<strong>Fellow</strong><br />
First Year<br />
<strong>Fellow</strong><br />
Second Year<br />
Post <strong>Fellow</strong>ship<br />
Experience<br />
cytogenetics and<br />
molecular laboratories).<br />
Has basic understanding<br />
<strong>of</strong> hospital budgetary<br />
issues that may be<br />
specific to<br />
hematopathology or<br />
pathology in general.
RESIDENT EVALUATION METHODS AND DOCUMENTATION<br />
in the Division <strong>of</strong> <strong>Hematopathology</strong><br />
A) <strong>Hematopathology</strong> Test for <strong>Resident</strong>s:<br />
1. Subject Material:<br />
Images, glass slides and/or laboratory data from areas <strong>of</strong> rotation that have<br />
been completed:<br />
o Bone marrow<br />
o Laboratory hematology<br />
o Lymph node<br />
2. Evaluation Method:<br />
Review information provided<br />
Examine glass slides and/or images with other laboratory/clinical data<br />
provided<br />
Select best diagnosis from list <strong>of</strong> choices<br />
3. Dates administered – Approximately 1½ weeks prior to completion <strong>of</strong> core<br />
hematopathology rotation blocks.<br />
B) Completion <strong>of</strong> Checklists according to rotation service (Bone Marrow, Lymph Node,<br />
Pediatric <strong>Hematopathology</strong>, Laboratory Hematology)<br />
C) <strong>Department</strong> <strong>of</strong> Pathology <strong>Resident</strong> Evaluation Forms (covering multiple competencies)<br />
D) Documentation <strong>of</strong> Observation and Performance <strong>of</strong> Bone Marrow Aspirates and Biopsies<br />
by Completion <strong>of</strong> Bone Marrow Performance Form<br />
E) Documentation <strong>of</strong> Conference Attendance at Journal Club, Interesting Case<br />
Conference, Wednesday morning <strong>Hematopathology</strong> Conference<br />
F) Faculty and staff observation <strong>of</strong> performance in conferences and sign-out and<br />
general observations regarding interpersonal relationships, communication skills and<br />
pr<strong>of</strong>essionalism, including utilization <strong>of</strong> departmental and extra-departmental resources<br />
G) Personal Meeting with Director or designate at end <strong>of</strong> core rotation segments
CONFERENCE SCHEDULE<br />
AND RESPONSIBILITIES
Monday<br />
CONFERENCE SCHEDULE<br />
12:00pm Seminars in Laboratory Medicine* Weekly CLB Room 1021<br />
Tuesday<br />
7:00am<br />
8:30 am<br />
9:15am<br />
11:00 am<br />
12:00pm<br />
12:00pm<br />
AP Didactic Conference/Unknown<br />
Surgical Pathology Slide<br />
Conference *<br />
SHY Adult Bone Marrow Review<br />
(BM2 Srvc <strong>Fellow</strong> or BM3 Srvc<br />
Pathologist)<br />
Continuing Education in Clinical<br />
Chemistry and Hematology<br />
Conference##, ***<br />
<strong>Hematopathology</strong> Flow<br />
Conference**<br />
<strong>Hematopathology</strong> Journal Club*,<br />
***,###<br />
Patient Safety & Risk Management<br />
in <strong>Hematopathology</strong> Conference*,<br />
###<br />
Weekly Totten Room, Scaife 618<br />
Weekly<br />
Weekly;<br />
Hematology<br />
alternates with<br />
Chemistry<br />
~Several<br />
times/month<br />
(see schedule)<br />
Every other<br />
week<br />
Every other<br />
week<br />
Teleconference from PUH<br />
G304 (412-864-7845)<br />
CLB Room 1021<br />
CLB Room 9018<br />
Totten Room, Scaife 618<br />
PUH G323<br />
7:00am CP Didactic Conference* Weekly CLB Room 1021<br />
Wednesday<br />
8:30am<br />
<strong>Hematopathology</strong> Conference<br />
(case presentation)* ***, ###<br />
Weekly Totten Room, Scaife 618<br />
12:00pm <strong>Department</strong> Research Seminar + Weekly PUH 11 th Floor<br />
Thursday<br />
12:00pm Shadyside Tumor Board# Intermittent<br />
8:00am Pediatric BM Conference +++ 2 nd and 4 th<br />
Thursday <strong>of</strong><br />
each Month<br />
12:00pm<br />
Anatomic Pathology Grand<br />
Rounds *<br />
Weekly<br />
4:00pm<br />
+++, ***<br />
CHP Tumor Board<br />
Leukemia Conference<br />
1 st Thursday <strong>of</strong><br />
the month<br />
Shadyside West Wing<br />
Auditorium<br />
Teleconference from PUH<br />
G306<br />
Room 1104 A & B Scaife Hall<br />
Rangos Res. Bldg -<br />
Lawrenceville<br />
* Attendance for residents required either based on this rotation or departmental expectations.<br />
** Attendance at this conference is strongly encouraged for trainees (but not required).<br />
*** The trainees will be expected to present at these conferences.<br />
**** Attendance is strongly encouraged when hematopathology cases are being presented (be sure you are<br />
getting email notifications).<br />
@ Attendance not expected.<br />
+ Attendance optional.<br />
++ Attendance is required for residents on laboratory medical rotation.<br />
+++ Attendance is expected for trainees doing pediatric hematopathology.<br />
# See description concerning attendance obligations.<br />
## Attendance required when on laboratory hematology rotation and encouraged when on other parts <strong>of</strong><br />
hematology rotation. One presentation required (see schedule).<br />
### Attendance required for fellows
RESIDENT AND FELLOW CONFERENCE RESPONSIBILITIES<br />
MONDAY<br />
12:00PM (Weekly)<br />
Seminars in Laboratory Medicine are case presentations/lectures by residents, fellows and<br />
faculty. See Conference Schedule from Laboratory Medicine Office for topics.<br />
TUESDAY<br />
7:00AM<br />
The AP Didactic Conference/Unknown Surgical Pathology Slide Conference takes place on<br />
Tuesdays from 7:00 to 9:00 AM in the Totten Room. The slides and clinical history for the<br />
conferences when they occur will be available at PUH, Shadyside and Magee for preview<br />
at least one week prior to the conference. The schedule is available on the resident website<br />
(http://residents.pathology.pitt.edu/default.aspx). <strong>Fellow</strong>s may attend; however it is not<br />
required and hematopathology duties take precedence. Breakfast is served.<br />
8:30AM<br />
SHY Adult Bone Marrow Review takes place Tuesdays from 8:30 – 9 am. A few cases selected<br />
by the Hematology/oncology attending and fellow are presented via teleconference using Go to<br />
Meeting (complete instructions posted in G304). The cases will be presented by the attending<br />
pathologist on BM service 3, or the hematopathology fellow on BM service 2 with the BM3<br />
attending pathologist serving as a mentor.<br />
9:15AM<br />
Continuing Education in Clinical Chemistry and Hematology Conference takes place<br />
Tuesdays at 9:15 -10:15am (following the residents' lectures). The location is usually CLB<br />
room 1021. Each resident on the laboratory hematology rotation will present once.<br />
The sessions are targeted toward technologist education. Discussion between<br />
pathologists/trainees is encouraged. Periodic updating <strong>of</strong> important basic concepts is<br />
welcome. Please seek input from Dr. Contis when planning your hematology presentation.<br />
11:00AM (several times/month)<br />
The Flow Cytometry Conference is a time when interesting flow cytometry cases selected by<br />
the technologists are reviewed and discussed by one <strong>of</strong> the hematopathologists. Some<br />
conferences are used for didactic presentations on a specific topic. Generally, other<br />
hematopathologists, residents and fellows attend. No preparation is required. See<br />
schedule for dates <strong>of</strong> conference. (CLB, Room 9018)<br />
12:00PM<br />
Approximately twice per month, residents doing <strong>Hematopathology</strong> will be asked to select one (1)<br />
current journal article for presentation at our Journal Club (see separate schedule). The<br />
selection should be <strong>of</strong> interest to the presenter and others in the group and needs to be<br />
approved by the faculty member or fellow responsible for that date (see schedule for<br />
dates and trainee/faculty assignments). If requested, the faculty member can also <strong>of</strong>fer<br />
suggestions for appropriate articles or <strong>of</strong>fer additional advice in making a selection. The<br />
faculty person or fellow will also choose and present an article. The articles should be<br />
emailed to the hematopathology secretary, Jessica Klimkowicz, no later than Thursday<br />
preceding the Journal Club.<br />
In terms <strong>of</strong> presentation, it is important to provide enough background information to help explain<br />
why the study was done and /or may be important and how it relates to what is already<br />
known. Any detailed discussion <strong>of</strong> methodology that may be required should be done
when the results are presented. When presenting the results, it is important to go over the<br />
various tables and figures in the paper and also point out any problems that may be<br />
presented in the data or methodology. Finally, the ultimate significance <strong>of</strong> the study given<br />
the results found should be discussed. PowerPoint presentations are not at all necessary<br />
and are discouraged!<br />
Everyone attending should also be prepared to comment on the above issues so that a lively<br />
discussion will ensue.<br />
On all other Tuesdays, there will be a Patient Safety and Risk Management Conference (see<br />
separate schedules). Cases are presented and discussed that could potentially raise<br />
patient safety and risk management issues. For example, cases that are particularly prone<br />
to diagnostic errors or cases <strong>of</strong> entities seen so infrequently that pathologists might not be<br />
familiar with them would be <strong>of</strong> particular interest. Cases where there have been any types<br />
<strong>of</strong> errors made within or outside our institution would also be <strong>of</strong> particular value. Trainees,<br />
along with faculty members, may bring cases to show.<br />
WEDNESDAY<br />
7:00AM<br />
CP Didactic Conference is a set <strong>of</strong> lectures covering all <strong>of</strong> laboratory medicine, some<br />
pertinent to hematopathology. The schedule is available on the resident website.<br />
(http://residents.pathology.pitt.edu/default.aspx).<br />
8:30AM<br />
The <strong>Hematopathology</strong> Conference is entirely the responsibility <strong>of</strong> our division. The purpose is<br />
to provide a forum to go over morphologic, immunophenotypic, karyotypic, and genotypic<br />
issues together with their clinical correlates. We present 5 cases each week at conference,<br />
ideally 3-bone marrow (including Children’s cases) and 2 lymph nodes. The conference<br />
responsibilities include:<br />
Trainees on the adult bone marrow service will consult with faculty signing out marrows no later<br />
than Friday and choose 3 interesting and /or educational marrows. As at least one<br />
pediatric marrow should be presented if there is a trainee on the service, trainees on the<br />
adult marrow service need to consult with the pediatric service to be sure that 3 bone<br />
marrows/PB/ATL cases are being presented. Please do not add additional cases if there<br />
are already 5.<br />
A. After cases are selected, please sign-up by giving name and number to the bone marrow<br />
technologist or leaving a voicemail message on the Audix line (802-3273). NAMES<br />
MUST BE SUBMITTED BY THE END OF MONDAY.<br />
The trainees will take images using one <strong>of</strong> the digital camera set-ups in the Division <strong>of</strong><br />
<strong>Hematopathology</strong> (room G304 conference room or G323 lymph node sign-out room) and<br />
find out the case history from the physician or the chart. The images should be imported<br />
into a PowerPoint presentation. At the conference, the trainees will present the case<br />
including a brief history, any prior material and any ancillary studies at the conference. In<br />
addition, some interesting aspects <strong>of</strong> the case may be discussed briefly (e.g. implications<br />
<strong>of</strong> an unusual morphologic appearance, significance <strong>of</strong> unusual phenotype). Don’t give a<br />
lecture. Cytogenetics will be presented by the cytogenetics laboratory faculty or by a<br />
trainee rotating in Cytogenetics. <strong>Fellow</strong>s are expected to present their own cytogenetic<br />
findings as long as the laboratory emails them the karyotypes/FISH images to show and a<br />
cytogeneticist can assist when needed. Genotypic results are usually presented by the<br />
Division <strong>of</strong> Molecular Diagnostics. If no trainee is on the service, the cases are presented
y the faculty member. Case presentations including all ancillary studies should be less<br />
than 8 minutes to allow time for discussion.<br />
B. Trainees on the lymph node service will consult with the faculty member signing out lymph<br />
nodes and select two lymph node cases to present. In the absence <strong>of</strong> appropriate current<br />
cases, older educational cases may be selected. The trainees will take images <strong>of</strong> the case and<br />
if at all possible get the history from the chart or physician. At the conference, using<br />
PowerPoint the trainee will present the case including a brief history, the pathology and any<br />
ancillary studies.<br />
C. Remember case presentations must be relatively brief, to the point and interesting both to<br />
pathology trainees and clinicians. If possible try to present cases in an interactive fashion.<br />
(Totten Room, Scaife 618)<br />
D. If there is >1 trainee on a service, the presenting obligations should be shared.<br />
Guidelines for PowerPoint Presentations<br />
1. Keep the presentation simple. Your time is better spent reading about the case<br />
rather than having multicolored images flying around.<br />
2. Try to limit the number <strong>of</strong> images shown- make sure that each image makes a point.<br />
3. Photomicrographs should occupy the entire screen.<br />
4. Please use images that are in focus.<br />
5. No more than two flow histograms should be on a single screen (or just use the<br />
electronic overhead projector).<br />
6. Up to 4 immunostains may be presented in a single screen. It is unnecessary to<br />
illustrate all immunostains- you need to choose critical ones or ones that are<br />
necessary to make your point. For example, please don’t show a series <strong>of</strong> 5<br />
negative stains.<br />
7. Laboratory results can either be discussed or, if presented on a screen, must have<br />
not only the numerical results, but also the units and preferably the normal ranges.<br />
The lab results that are presented visually do not need to include every single<br />
laboratory test that was done on the patient.<br />
8. In at least most cases, there is no need to present a written bone marrow<br />
differential.<br />
12:00PM<br />
Trainees are encouraged, but not required to attend the <strong>Department</strong>al Research Conference.<br />
12:00PM<br />
UPMC-Shadyside Tumor Board. Cases are presented by the hematopathology fellow or if<br />
necessary by a hematopathology faculty member at the request <strong>of</strong> a clinician (detailed<br />
procedure on page 22) (Shadyside West Wing Auditorium). <strong>Resident</strong>s do not attend.<br />
THURSDAY<br />
8:00AM<br />
A Pediatric Bone Marrow Case Conference is held via teleconference to the Children’s<br />
Hospital, Lawrenceville except for the first Thursday <strong>of</strong> the month. This is attended<br />
primarily by the pediatric hematology/oncology fellows, and the pathology trainees on the<br />
pediatric bone marrow service. The bone marrow technologists will collect the cases from<br />
the prior week to be presented by the attending hematopathologist on service. <strong>Fellow</strong>s
may be expected to present the cases. Complete instructions for using Go-To-Meeting are<br />
in the pediatric bone marrow signout room G304,<br />
12:00PM<br />
<strong>Resident</strong>s doing hematopathology are expected to attend Anatomic Pathology Grand Rounds.<br />
4:00PM<br />
Pediatric Leukemia Tumor Board is held the first Thursday <strong>of</strong> the month from 4:00 to 5:00 in<br />
Conference Rooms B123 and B124, Floor B, at Children’s Hospital <strong>of</strong> Pittsburgh. The<br />
hematopathologists and bone marrow technologists are notified by e-mail the week <strong>of</strong> the<br />
conference as to which cases are to be presented. The bone marrow technologists will<br />
collect the cases for the trainees. The trainees will capture images and prepare a<br />
PowerPoint presentation. The presentations should be brief and succinct and include 1<br />
slide <strong>of</strong> peripheral smear, 1-2 slides <strong>of</strong> aspirate, and 1-2 slides <strong>of</strong> biopsy as well as any<br />
pertinent cytochemical or immunohistochemical stains. Flow images can also be presented<br />
– please check with attending regarding specific images to present. Pertinent results from<br />
the cytogenetics and/or molecular studies should be summarized in 1-2 slides. The<br />
presentation should be approved by the attending prior to presentation.
Additional information regarding Shadyside Tumor Board Conference<br />
Wednesday Noon<br />
Contact Person SHY: Melissa Forkey 412-648-6466<br />
Contact Person PUH: Celina Fortunato 412-647-0263<br />
1. UPMC – Shadyside will call the UPMC – Presbyterian bone marrow lab at 647-<br />
0263 with a list <strong>of</strong> patient names NO LATER THAN the Friday preceding the<br />
conference (preferably earlier).<br />
2. The Bone Marrow Technologist will inform the presenter.<br />
a. First inform the 2 nd year fellow if there is one. If the 2 nd year fellow cannot<br />
present (i.e. on a rotation that precludes their availability), the fellow must<br />
inform the bone marrow technologists immediately. Go to step b.<br />
b. Next, inform the 1 st year fellow. If the 1 st year fellow cannot present (i.e. on a<br />
rotation that precludes their availability), the fellow must inform the bone<br />
marrow technologist immediately. Next inform the second 1 st year fellow if<br />
there is one. If there is more than one second year fellow or more than one firstyear<br />
fellow, please ask the one not on a diagnostic signout service first to do<br />
the tumor board (unless they are on another service that would preclude their<br />
being able to attend). If this fellow also cannot present, go to step c.<br />
c. If no fellow can present, the bone marrow technologist will inform the<br />
pathologist on the lymph node service for the week it is to be presented.<br />
This way the bone marrow technologist will know who exactly will be presenting the<br />
conference.<br />
3. The bone marrow technologist will pull the slides and print the reports.<br />
4. If surgical pathology cases are requested, the bone marrow technologist will notify<br />
Melissa Forkey who will make other arrangements for the cases to be presented by<br />
surgical pathologist.<br />
5. The bone marrow technologist will inform the person responsible for the<br />
presentation – prior to the weekend – that the cases are ready.<br />
6. The bone marrow technologist will record on the log sheet that the cases are ready<br />
and the presenter is notified.<br />
The bone marrow technologist will call Melissa Forkey and inform her who will be presenting the case.
Pediatric <strong>Hematopathology</strong><br />
and General/Special<br />
Hematology Laboratory<br />
Experience
Pediatric <strong>Hematopathology</strong> and General/Special Hematology Laboratory<br />
Experience (3 weeks)<br />
Trainees should report to the pathologist signing out CHP bone marrows. The pathologist<br />
covering the general hematology laboratory (“ATL” service) is usually the same person; if this<br />
person is different from the one signing out CHP bone marrows, then contact him or her also.<br />
Also, trainees should touch base with Dr. Contis regarding their continuing education<br />
conference presentation at the beginning <strong>of</strong> their rotation. <strong>Resident</strong>s also will discuss with Dr.<br />
Contis or her designee how to best apportion their time and how to gain in-laboratory<br />
experience with the technologists.<br />
Pediatric <strong>Hematopathology</strong> Experience<br />
1. Review Blood Cell Morphology. (Published by the ASCP). RBC, WBC, Normal and Abnormals<br />
(Binders with CDs available in G304 and slides are also on resident’s shared drive, under “CP<br />
Didactic Lectures\Previous CP Didactic Lectures\Hemepath\ASCP Hematology Images.” Each set<br />
is a separate PowerPoint file and the key and reading material for all 6 sets <strong>of</strong> images is in the<br />
PDF).<br />
2. In the first week, touch base with the lead Celina Fortunato to schedule a teaching session<br />
with the bone marrow technologists (typically Tuesday afternoon is best) to review<br />
peripheral blood and bone marrow aspirate smears<br />
3. Pediatric Bone Marrow Sign out.<br />
A. Preview and sign out cases with hematopathology faculty in a fashion analogous to<br />
procedures followed with adult bone marrows.<br />
• Meet with faculty on service to coordinate these activities.<br />
• See also “Policy for sign-out <strong>of</strong> CHP marrows that have biopsies”.<br />
4. Review <strong>of</strong> specimens from Automated Testing Laboratory and Flow Cytometry Laboratory<br />
A. Preview and sign out pediatric and adult abnormal peripheral blood films and body fluid slides<br />
sent for review with faculty hematopathologist. Gather relevant clinical and pathology<br />
information (such as cytology results) prior to sign-out.<br />
B. If there is a case with interesting findings, please give the slide and copy <strong>of</strong> the ATL review<br />
sheet to Dr. Roth in order to contribute to the study sets.<br />
5. Review <strong>of</strong> selected cases <strong>of</strong> Hemoglobinopathies with the CAP textbook<br />
Trainees should pre-review the HPLC tracings that are being faxed weekly to our division<br />
and they should contact Dr. Dobrowolski and meet with him once to review the cases with<br />
him.<br />
6. Review <strong>of</strong> educational materials<br />
A. Swerdlow SH, Collins RD Pediatric <strong>Hematopathology</strong>, Churchill Livingstone, 2001.
B. Nathan, DG and Orkin, SH, Nathan and Oski’s Hematology <strong>of</strong> Infancy and Childhood, 7 th<br />
Edition, WB Saunders, 2009.<br />
C. Penchansky L, Pediatric Bone Marrow, Springer, 2004.<br />
D. Glassy EF. Color Atlas <strong>of</strong> Hematology, CAP 1998<br />
E. Peripheral smear and fluid slide study sets are available for checkout from Office Secretary –<br />
G306.<br />
F. Chromogram information for hemoglobinopathy information (in supplemental materials)<br />
G. Bain BJ. Haemoglobinopathy Diagnosis, 2 nd ed. Blackwell, 2006.<br />
H. Schuman GB, Friedman SK. Wet Urinalysis, ASCP, 2003.<br />
I. Galagan, K. Color Atlas <strong>of</strong> Body Fluids: An Illustrated Field Guide Based on Pr<strong>of</strong>iciency<br />
Testing.<br />
J. Proytcheva, M.A. (Ed), Diagnostic Pediatric <strong>Hematopathology</strong>, 2011.<br />
7. Review results <strong>of</strong> ancillary procedures performed on marrows you have reviewed and read<br />
addenda faculty have issued.<br />
Policy for sign-out <strong>of</strong> CHP marrows that have biopsies<br />
1. <strong>Hematopathology</strong> signs out biopsies on hematologic disease cases including non-Hodgkin<br />
lymphomas. CHP surgical pathology signs out the biopsies on tumor cases.<br />
2. On all cases with a biopsy, the histologic section must be reviewed by the hematopathologist<br />
even if it is not a case where the hematopathologist is signing out the biopsy. This is to ensure<br />
that it does not conflict with the aspirate smear evaluation.<br />
3. In all cases with a biopsy, where CHP is signing out the biopsy the hematopathologist should<br />
correlate with the CHP pathology report to make sure that the two diagnoses will not conflict. In<br />
some cases, this may involve contacting the CHP pathologist and jointly reviewing the case.<br />
Policy for Assignment <strong>of</strong> Marrow Cases without Biopsies<br />
Marrow sign-out is the responsibility <strong>of</strong> the faculty/trainee on the service when the marrow biopsy<br />
typically would come out. However, all cases done on Friday must be pre-reviewed by those on<br />
the service that day. In addition, all marrows, pediatric or adult, with or without flow cytometry that<br />
do not have a biopsy and are received before noon on a Friday, must be signed out by those on<br />
the service that week. They are not the responsibility <strong>of</strong> those on the following week. Children’s<br />
bone marrow aspirates with biopsies for metastatic tumor evaluation received on Friday will be the<br />
responsibility <strong>of</strong> the pathologist on service the following week, even though we are only signing out<br />
the aspirate.
General/Special Hematology Experience<br />
1. Meet with Dr. Contis or her designee to review plan for completion <strong>of</strong> checklist that follows.<br />
2. Plan laboratory presentation (see instructions on Continuing Education in Clinical Chemistry<br />
and Hematology).<br />
3. See checklist for specific activities and educational resources.
General/Special Hematology Laboratory Experience Checklist [Revised 10/2013]<br />
This checklist indicates the areas that need to be covered during the general/special laboratory<br />
experience and the specific activities that are to be performed. This should occupy approximately half<br />
your time when combined with pediatric hematopathology (as in core resident/fellow rotation).<br />
Check boxes to the left <strong>of</strong> specific activities when the indicated activities are completed<br />
This checklist is also included separately and must be signed by the resident/fellow and turned in to<br />
Dr. Swerdlow’s <strong>of</strong>fice at the completion <strong>of</strong> the rotation.<br />
1. General Activities<br />
Review a spectrum <strong>of</strong> abnormal peripheral blood and body fluid results. These should include:<br />
o<br />
o<br />
o<br />
o<br />
o<br />
o<br />
Macrocytic and microcytic anemias<br />
RBC changes in autoimmune hemolytic anemia<br />
Thrombocytopenia and inherited platelet disorders<br />
Granulocytosis and granulocytopenia<br />
Blasts in the peripheral blood<br />
Abnormal cytoplasmic inclusions in WBC & RBC as available, either as review specimens or in<br />
the study sets<br />
If cases <strong>of</strong> each <strong>of</strong> the above are not available and you are in your last week, be sure to review<br />
teaching slides or other resources (ask Dr. Contis or designee for help if required).<br />
Follow up on patients whose abnormal peripheral bloods and body fluids you have reviewed to<br />
determine the significance <strong>of</strong> the abnormality on diagnostic and therapeutic decision making and<br />
ultimate clinical outcome.<br />
Keep a list <strong>of</strong> the following (include relevant clinical information that you have obtained for each<br />
patient):<br />
The laboratory tests (routine and special) that you have observed (or reviewed following<br />
returns by the reference laboratories).<br />
The abnormal peripheral blood films and body fluid smears that you have seen. Also see<br />
section 2B below.<br />
Plan laboratory hematology presentation for Continuing Education in Clinical Chemistry and<br />
Hematology (see detailed instructions in <strong>Resident</strong>/<strong>Fellow</strong> Handbook).<br />
2. Specific Activities
General Hematology Laboratory (ATL)<br />
The hematology lead technologist, Betty Austin will help you with orientation to hematology<br />
instrumentation.<br />
A. General<br />
Review procedure manuals in General Hematology, Coagulation and Urinalysis<br />
including the procedures for operating the Iris iQ 200 urinalysis instrument. The<br />
purpose <strong>of</strong> this review is to learn how a procedure manual is constructed, to<br />
appreciate the CLSI (formally NCCLS) format and to learn how to find a procedure<br />
when needed.<br />
Be sure that the Laboratory Hematology Staff Meeting fellow attendance sheet is<br />
completed with your signature at all the staff meetings/laboratory management<br />
meetings that you attend.<br />
Participate in troubleshooting. This includes analysis <strong>of</strong> problems with function <strong>of</strong><br />
instruments, quality control, or patient data that appear spurious. The problems will<br />
be brought to your attention by the technical staff and/or Dr. Contis or designee.<br />
B. Hematologic Microscopy<br />
Review normal PB morphology, understanding variations between adult and pediatric<br />
values. (Review ASCP sets located in room G315, if not previously reviewed or if further<br />
review is needed).<br />
• See criteria for evaluating red cells on sheet “Red Cell Morphology Classification<br />
(1+, 2+, 3+)” in the hard copy supplement.<br />
Evaluate all abnormal slides referred to the pathologist (ongoing throughout rotation). This<br />
is performed by checking to see if there are slides for review (bone marrow technologists<br />
will usually bring them to you). When slides are designated, the trainee should obtain<br />
clinical information about the patient, including checking CoPath for prior pathologic<br />
evaluations, then review the slide including performing a 100-200 cell differential for<br />
peripheral blood films and a morphologic review <strong>of</strong> the cytospin slide for a fluid. The<br />
trainee should then formulate a differential diagnosis and then review the case with the<br />
pathologist on the laboratory service (see schedule). This review is an essential part <strong>of</strong> the<br />
laboratory’s function since the ATL lab is <strong>of</strong>ten the first place where an abnormality is<br />
detected.<br />
Peripheral Blood Films<br />
Body Fluid Cytospin slides<br />
Correlate body fluid results from cytology laboratory with hematology findings.
Alert Dr. Christine Roth to interesting peripheral blood films or body fluid slides. Provide<br />
her the slides, a photocopy <strong>of</strong> the lab values and clinical information.<br />
C. Automated Equipment: Coulter DXH800, Cellavision<br />
Review information provided in your folder<br />
Review procedure manual<br />
Observe:<br />
Setup/Cleaning <strong>of</strong> instrument<br />
QC/QA Procedures, graphing<br />
Operation <strong>of</strong> Coulter and Cellavision<br />
Understand principles <strong>of</strong> instrument. This is discussed in procedure manual and also lead<br />
techs can answer questions.<br />
Review interpretation <strong>of</strong> Coulter dot plots and causes <strong>of</strong> spurious results. Use procedure<br />
manual as well as cases you observe in the laboratory.<br />
• See examples on the “Complete Blood Count” in the hard copy supplement.<br />
• See 2 slides explaining histograms and dot plots.<br />
• See up-to-date, “Automated Hematology Instrumentation”.<br />
• Learn to evaluate normal and abnormal patterns.<br />
• Understand meaning <strong>of</strong> individual flags and mechanisms for evaluating flags.<br />
• Know what a flag is.<br />
• Review CAP & Instrumentation QC/QA date.<br />
Urinalysis<br />
1. AuWi Siemens:<br />
Review information provided in your folder<br />
Review procedure manual<br />
Observe set-up and urinalysis runs<br />
Observe:<br />
Set up, preparation <strong>of</strong> reagents, samples
QC/QA Procedures, graphing<br />
Running, evaluation <strong>of</strong> samples<br />
Understand principles <strong>of</strong> instrument and manual back-up procedures (when and how)<br />
Understand sensitivities and specificity <strong>of</strong> color reactions and drug interference.<br />
Understand principles <strong>of</strong> instrument and back-up procedures (when and how).<br />
Review Educational Resources<br />
Crystals and casts<br />
Cells<br />
2. Coagulation automated equipment: STA-R Evolution (Diagnostica Stago, Inc)<br />
Review procedure manual<br />
Observe:<br />
Set up, preparation <strong>of</strong> reagents, samples<br />
QC/QA Procedures, graphing<br />
Running, evaluation <strong>of</strong> samples<br />
Understand significance <strong>of</strong> results for pre-operative screening and monitoring<br />
anticoagulant therapy by reading text materials or original literature and by discussion<br />
<strong>of</strong> issues with the pathologist on the laboratory service. This should include<br />
understanding the significance and use <strong>of</strong> the INR.<br />
Observe the D-dimer procedure and understand its clinical usage as a negative<br />
predictor for DVTs and as a positive indicator <strong>of</strong> DIC.<br />
Administrative Aspects <strong>of</strong> Laboratory<br />
Attend the weekly Hematology Laboratory meeting during your 3-week rotation.<br />
When: Every other Wednesday at 10:00 AM<br />
Where: CLB 3 rd Floor Room 3018
If the resident’s rotation includes the last week <strong>of</strong> the month, they can attend the<br />
Shadyside Laboratory Operation meeting at 8:30 AM, at Shadyside Hospital the last<br />
Tuesday <strong>of</strong> each month in Room 2.15 (ground floor – West wing elevators). Contact<br />
Dr. Contis for information.<br />
3. Special Hematology Laboratory<br />
Many tests are sent out to reference laboratories.<br />
A. General<br />
Review test menu and procedure manual<br />
• Observe any test procedures being performed.<br />
B. Specific Tests:<br />
• Hemoglobin Evaluation<br />
Understand co-migration <strong>of</strong> hemoglobins and methods for distinguishing them, clinical<br />
significance, relationship to Sickledex.<br />
Understand mechanisms <strong>of</strong> false positives/negatives, effect <strong>of</strong> transfusion on findings.<br />
Review HPLC Examples for Variant Hemoglobins, shelved in G315 under Hematology<br />
References.<br />
Review Color Atlas <strong>of</strong> Hemoglobin Disorders: A compendium based on Pr<strong>of</strong>iciency Testing<br />
(located in G315).<br />
Understand principles <strong>of</strong> HPLC for hemoglobin separation.<br />
Review and interpret tracings that come back from the reference laboratories and from<br />
CHP Laboratories.<br />
Review chromogram information for hemoglobinopathy information (in supplemental<br />
materials)<br />
Contact Dr. Dobrowolski at CHP to arrange a time for review <strong>of</strong> HPLC and follow-up study<br />
results. <strong>Resident</strong>s are expected to meet with Dr. Dobrowolski twice at CHP.
• Test <strong>of</strong> RBC function<br />
Know how these tests are performed and how they are useful:<br />
RBC enzymes (G6PD, PK, GPI, etc.)<br />
Teaching case /recent send out file<br />
Read about<br />
Plasma, serum, urine, hemoglobin<br />
Teaching case /recent send out file<br />
Read about<br />
Osmotic fragility<br />
Teaching case /recent send out file<br />
Read about<br />
Heinz bodies<br />
Teaching case /recent send out file<br />
Read about<br />
• Miscellaneous Tests<br />
Acetylcholinesterase (ACHE)<br />
Muramidase<br />
Urine, serum myoglobin<br />
Flow cytometry for PNH (If not reviewed in Flow rotation)<br />
Neutrophil oxidative product formation analysis (If not reviewed in Flow rotation.)<br />
• Staining <strong>of</strong> bone marrow smears<br />
Routine Stains<br />
Cytochemical and immunocytochemical methods/procedures
• Other Hematology Testing: (Vitamin B12, serum and RBC folate, serum iron<br />
and ferritin)<br />
These tests are now done in chemistry.<br />
Review how they are used in the workup <strong>of</strong> hematologic disorders.<br />
References:<br />
Color Atlas <strong>of</strong> Hemoglobin Disorders, A Compendium Based on Pr<strong>of</strong>iciency<br />
Testing. James D. Hoyer and Steven H. Kr<strong>of</strong>t, Editors, 2003.<br />
Clinical Diagnosis and Management by Laboratory Methods, 22nd Edition, John<br />
Bernard Henry, 2007<br />
Haemoglobinopathy Diagnosis, 2 nd edition. Barbara Bain, Blackwell Science, 2006.<br />
I have completed the Pediatric <strong>Hematopathology</strong> Checklist<br />
Name __________________________________________ (printed)<br />
__________________________________________ (signature)<br />
Date<br />
__________________________________________
HEMOGLOBIN ANALYSIS CASE LOG<br />
ACCESSION NUMBER<br />
DIAGNOSIS
PB AND FLUID REVIEW LOG<br />
ACCESSION NUMBER<br />
DIAGNOSIS
PB AND FLUID REVIEW LOG<br />
ACCESSION NUMBER<br />
DIAGNOSIS
Continuing Education in Clinical Chemistry and Hematology<br />
Description:<br />
The Continuing Education Conference is organized around a case discussion (resident) and<br />
scientific issue or laboratory management/quality assurance presentation (faculty). A method or<br />
technical issue may also be presented by a lead technologist. Coordination between the 2-3<br />
presenters is encouraged. The sessions are targeted toward technologist education. Discussion<br />
between pathologists/trainees and technologists is encouraged. Periodic updating <strong>of</strong> important<br />
basic concepts is welcome. Please seek input from Dr. Contis, or another hematopathologist when<br />
planning your hematology presentation.<br />
Day, time and location:<br />
Tuesdays at 9:15 -10:15 AM (following the residents' lectures), unless the resident lectures times<br />
are changed. The location is usually CLB Room 1021.<br />
Frequency <strong>of</strong> presentation: Each resident on the laboratory hematology rotation will present<br />
once, usually on the last week <strong>of</strong> their rotation.<br />
Length <strong>of</strong> each presentation: 15-20 minutes<br />
Examples <strong>of</strong> Topics:<br />
1. Case discussion (<strong>Resident</strong> or fellow).<br />
a. “Atypical lymphocytes,” 9/4/07, Dr. A. Henry<br />
b. “Case <strong>of</strong> G6PD deficiency,” 9/25/07, Dr. I Batal<br />
c. “Identification <strong>of</strong> urinary crystals,” 10/16/07, Dr. M. Rollins-Raval<br />
d. “Nucleated Red Blood Cells and the Coulter LH 750 and the Sysmex XE 2100,”<br />
12/16/08, Dr. Hannah Kastenbaum<br />
e. "Scaling the Peaks: High-Performance Liquid Chromatography And the Detection <strong>of</strong><br />
Hemoglobin S", 5/5/09, Dr. Milon Amin<br />
f. “Cystine crystals,” 12/12/10, Dr. Kelley Garner<br />
2. Method or technical issue (Lead technologist or other)<br />
a. “Validation <strong>of</strong> reference ranges for automated ESR method.” 9/4/07, E. Austin<br />
b. “Review and tips for making thick smears for blood parasites,” P. Nowack<br />
c. “Review <strong>of</strong> PFA-100,” 9/9/08, Dr. S. Monaghan<br />
d. “Review <strong>of</strong> Bronchoalveolar Lavage (BAL) Analysis for Cell Counts & Differentials,<br />
12/2/08,” Dr. S. Gibson<br />
e. “A Proposed Plan for the Comparison Evaluation <strong>of</strong> the Coulter DxH 800, Sysmex<br />
XE, and Coulter LH 750,” Dr. S. Monaghan<br />
3. Scientific issue, quality assurance or management (Faculty)<br />
a. Discuss a method, disease, recent literature, QA study, etc.<br />
b. “Immature reticulocyte fraction,” 10/21/08,” Dr. L. Contis<br />
c. “Automated Cell Counts on Pleural Fluid: Review <strong>of</strong> a Study on the Sysmex XE-<br />
2100, 12/2/08,” Dr. R. Felgar.<br />
d. “Platelet Counting by the Coulter LH 750 and Sysmex XE 2100,” 12/16/08, Dr. S.<br />
Monaghan
Pediatric <strong>Hematopathology</strong> Checklist<br />
Note: Items indicated in BOLD constitute the basic knowledge expected <strong>of</strong> residents<br />
rotating in <strong>Hematopathology</strong>. Additional (non-bolded) items should also be included for<br />
advanced learners including fellows.<br />
Orientation with Hematopathologist on Pediatric <strong>Hematopathology</strong> service.<br />
Knows how normal pediatric blood and bone marrow differ from those <strong>of</strong> adults.<br />
Knows special aspects <strong>of</strong> neonatal hematopathology (see K. Foucar, “Neonatal<br />
<strong>Hematopathology</strong>: Special Considerations” in Collins RD and Swerdlow SH, eds. Pediatric<br />
<strong>Hematopathology</strong>).<br />
Knows role <strong>of</strong> cytogenetic and molecular diagnostic studies in evaluating hematopoietic /<br />
lymphoid disorders in bone marrow and blood.<br />
Learn diagnostic criteria using multiparameter approach and clinical implications for each <strong>of</strong><br />
the following:<br />
Diagnosis / Finding<br />
NEOPLASTIC DISORDERS<br />
Observed<br />
Actual Case<br />
Observed<br />
Teaching<br />
File Case<br />
Read About<br />
Acute Lymphoblastic Leukemia<br />
B-lymphoblastic leukemia / lymphoma<br />
T- lymphoblastic leukemia / lymphoma<br />
Acute Myeloid Leukemias<br />
Acute Myeloid Leukemia with 11q23 abnormality<br />
Acute Myeloid Leukemia with recurrent<br />
cytogenetic abnormality, other<br />
Acute Megakaryoblastic Leukemia<br />
AML/MDS associated with congenital marrow<br />
failure syndromes<br />
Acute Myeloid Leukemia, not otherwise<br />
specified<br />
Mixed lineage acute leukemia<br />
Myeloid proliferations associated with Down<br />
Syndrome<br />
Acute Myeloid Leukemia<br />
Transient Abnormal Myelopoiesis
Chronic Myeloproliferative Neoplasms<br />
Chronic Myelogenous Leukemia<br />
Myelodysplastic/Myeloproliferative Disease<br />
Juvenile Myelomonocytic Leukemia<br />
<br />
<br />
Myelodysplastic Syndromes<br />
Childhood Myelodysplastic Syndromes<br />
Refractory cytopenia <strong>of</strong> childhood<br />
Mature Lymphoid Neoplasms<br />
Burkitt Lymphoma<br />
Diffuse Large B-cell Lymphoma<br />
Anaplastic Large Cell Lymphoma, ALK+<br />
Pediatric Nodal Marginal Zone Lymphoma<br />
Pediatric Follicular Lymphoma<br />
Systemic EBV+ T-cell LPD <strong>of</strong> Childhood<br />
Hydroa Vacciniforme-like Lymphoma<br />
Nodular Lymphocyte Predominant Hodgkin<br />
Lymphoma<br />
Classical Hodgkin Lymphoma<br />
Histiocytic Neoplasms<br />
Langerhans cell histiocytosis<br />
Mast Cell Disease<br />
Metastatic Tumors<br />
Neuroblastoma<br />
Rhabdomyosarcoma<br />
Ewing sarcoma<br />
NON-NEOPLASTIC DISORDERS<br />
Congenital Disorders/Syndromes with Prominent<br />
Hematologic Abnormalities (Hereditary bone<br />
marrow failure)<br />
Fanconi Anemia
Diamond Blackfan Syndrome<br />
Congenital Dyserythropoietic Anemia<br />
Congenital Sideroblastic Anemia<br />
Pearson Syndrome<br />
Severe Congenital Neutropenia/Kostmann’s<br />
Syndrome<br />
Cyclic Neutropenia<br />
Shwachman-Diamond Syndrome<br />
Dyskeratosis Congenita<br />
Reticular Dysgenesis<br />
May-Hegglin Anomaly<br />
Bernard-Soulier Syndrome<br />
Wiskott-Aldrich Syndrome<br />
Congenital Amegakaryocytic Thrombocytopenia
Familial Platelet Syndrome with Predisposition to<br />
Acute Myeloid Leukemia<br />
X-linked thrombocytopenia<br />
Thrombocytopenia with Absent Radii (TAR)<br />
Gray Platelet Syndrome<br />
X-Linked Lymphoproliferative Disorder<br />
DiGeorge Syndrome<br />
Severe Combined Immunodeficiency Disease<br />
Red Cell cytoskeletal/membrane abnormalitieshemolytic<br />
anemia<br />
Red Cell enzyme deficiencies-hemolytic anemia<br />
Hemoglobinopathies<br />
Sickle cell disease<br />
Thalassemia<br />
Other<br />
Gaucher Disease<br />
Niemann-Pick<br />
Mucopolysaccharidoses<br />
Other Anemias and Conditions<br />
Transient erythroblastopenia <strong>of</strong> childhood<br />
Alloimmune hemolytic anemia <strong>of</strong> newborn<br />
Iron deficiency<br />
B12/folate deficiency<br />
Parvovirus<br />
Thrombocytopenia (see also above)<br />
Autoimmune Thrombocytopenic Purpura<br />
TTP/Hemolytic Uremic Syndrome<br />
Congenital syndromes
Drugs/toxins<br />
Infection-associated<br />
Thrombocytosis<br />
Neutropenia<br />
Immune neutropenia<br />
Neutrophilia/Leukemoid Reaction<br />
Infection/inflammatory disorders<br />
Medications<br />
Eosinophilia<br />
Basophilia<br />
Lymphopenia<br />
Infection<br />
Medication<br />
Autoimmune<br />
Nutritional Deficiency<br />
Congenital Disorders<br />
Lymphocytosis<br />
Epstein Barr Virus (infectious mononucleosis)<br />
Pertussis<br />
Infection, other<br />
Granulomas<br />
Myel<strong>of</strong>ibrosis
Aplastic Anemia<br />
Idiopathic Drugs<br />
Secondary<br />
Infection Associated<br />
Drugs/toxins<br />
Hemophagocytic/Lymphohistocytic Syndromes<br />
Familial lymphohistiocytosis<br />
Secondary hemophagocytic/macrophage<br />
activation syndrome<br />
Immunodeficiencies<br />
Autoimmune Lymphoproliferative Syndrome<br />
Other Primary Immunodeficiencies (see<br />
above)
PRESENTED THE FOLLOWING PEDIATRIC BONE MARROW OR LYMPH NODE CASES<br />
(<strong>Fellow</strong>s should submit presentation in portfolio)<br />
PHB NUMBER<br />
DIAGNOSIS
UTILIZED THE FOLLOWING BONE MARROW RESOURCES<br />
Swerdlow SH, Collins RD Pediatric <strong>Hematopathology</strong>, Churchill Livingstone, 2001.<br />
Orkin, SH, et al., Nathan and Oski’s Hematology <strong>of</strong> Infancy and Childhood, 7 th Edition.<br />
Penchansky L, Pediatric Bone Marrow, Springer, 2004.<br />
NOTE: Reading is an important component <strong>of</strong> this rotation. It is recognized that not all <strong>of</strong> the<br />
above resources can be used nor can most be read in entirety. Use <strong>of</strong> electronic and other<br />
resources to find and read up-to-date journal articles is also critical.<br />
I have completed the Pediatric <strong>Hematopathology</strong> Checklist.<br />
Name __________________________________________ (printed)<br />
__________________________________________ (signature)<br />
Date<br />
__________________________________________
UPMC PRESBYTERIAN SHADYSIDE<br />
Automated Testing Laboratory<br />
Pathologist Review Form<br />
Patient Name /Medical Record #<br />
Hospital: PUH/CLB SHY CHP MWH Date:<br />
Patient Diagnosis:<br />
Specimen Type: Peripheral blood smear Pleural fluid Peritoneal fluid CSF Other<br />
Tech Name/ Tech comments:<br />
PATHOLOGIST COMMENTS:<br />
BLID=Blasts are identified<br />
RMCP=Reactive mesothelial cells present<br />
FCSR=Flow cytometry studies recommended GTCBM=Correlation with pending bone<br />
marrow evaluation recommended<br />
AUERP=Auer rods present<br />
INCP=Inflammatory cells present<br />
CBCLDF=Atypical lymphoid population,<br />
worrisome for lymphoproliferative disorder.<br />
Recommend flow cytometric evaluation if clinically<br />
indicated<br />
GTAMC=Clusters <strong>of</strong> atypical mononuclear cells,<br />
worrisome for malignancy, correlation with<br />
cytology recommended<br />
GTPLC=Plasma cells identified, correlation with<br />
flow cytometry studies recommended<br />
Additional Pathologist comments:<br />
BACIE= Intracellular and extracellular bacteria<br />
present<br />
Correct the Differential? Yes / No<br />
Pathologist comment to tech:<br />
Stain quality: Satisfactory / Unsatisfactory<br />
RESIDENT/FELLOW :<br />
PATHOLOGIST :
Children’s Hospital <strong>of</strong> Pittsburgh <strong>of</strong> UPMC<br />
AUTOMATED TESTING LABORATORY<br />
412-692-9836<br />
PATHOLOGIST REVIEW<br />
HEMATOPATHOLOGY DEPARTMENT<br />
Name/Medical Record #:<br />
Date/Accession #:<br />
Patient Diagnosis<br />
Peripheral Blood Smear: Yes / NO<br />
Fluid type: CSF, Synovial, Pleural, Peritoneal, Pericardial, Bronchial Lavage<br />
TECHNOLOGIST COMMENT:<br />
TECH SUBMITTING REVIEW:<br />
PATHOLOGIST COMMENT: To be entered into Sunquest<br />
SEE CORRECTED DIFF <br />
<br />
PATHOLOGIST COMMENT: For Technologist only<br />
REVIEW RESIDENT/FELLOW:<br />
REVIEW PATHOLOGIST:
Clinical Experience in<br />
Hematology
Clinical Experience in Hematology at UPMC Shadyside<br />
Clinical Hematology / Performance <strong>of</strong> Bone Marrow Experience<br />
All activities are based at Hillman Cancer Center, 2 nd Floor unless indicated otherwise. Upon arrival, contact<br />
the on site bone marrow technologist who can help orient you to the facility and facilitate your opportunity to<br />
learn how to perform bone marrow examinations. The bone marrow technologist can either be found in room<br />
A220.36 or paged at (412) 958-8812 or the in-house pager 11443. This can also be facilitated by your seeing<br />
Celina Fortunato, in G325 or one <strong>of</strong> the bone marrow technologists who can call over to UPMC-Shadyside<br />
the week before you go over there.<br />
MONDAY TUESDAY WEDNESDAY<br />
AM Dr. Redner Clinic<br />
(Hillman Cancer Center,<br />
2 nd Floor Conference Rm.<br />
One).<br />
Learn to perform bone<br />
marrow aspirates /biopsies<br />
with Physician’s Assistant,<br />
Chris Lindberg, or other<br />
Physician’s Assistant<br />
Go with Bone Marrow<br />
Technologist on all marrows if<br />
Chris Lindberg, or other<br />
Physician’s Assistant with<br />
bone marrows to perform are<br />
not available<br />
Dr. R. Smith Clinic, Area A,<br />
Pod 1<br />
<br />
<br />
Dr. Kiss/Dr. Bontempo’s<br />
Hematology Clinic, Area A,<br />
Pod 3, Rooms 3B,C,D<br />
PM After Dr. Redner’s clinic is<br />
done, go with Bone<br />
Marrow Technologists on<br />
all marrows.<br />
<br />
<br />
Dr. Raptis/Dr. Agha’s<br />
Clinic, 3rd Floor, Hillman<br />
Cancer Center, Room 16<br />
Learn to perform bone<br />
marrow aspirates /biopsies<br />
with Chris Lindberg, or other<br />
Physician’s Assistant<br />
Begin UPMC-Presbyterian based<br />
flow cytometry laboratory<br />
rotation.<br />
Report to:<br />
Clinical Laboratory Building<br />
9 th Floor, Room 9033<br />
3477 Euler Way<br />
Pittsburgh, PA 15213<br />
412-864-6180<br />
Objectives:<br />
<br />
<br />
<br />
Learn how clinical hematology is practiced including patient concerns and what clinicians need from<br />
pathologists.<br />
Know how to perform bone marrow biopsies and aspirates including actually performing preferably at<br />
least 2 procedures.<br />
Know how bone marrow specimens are processed.
Performance <strong>of</strong> Bone Marrow<br />
Aspirations & Biopsies
Performance <strong>of</strong> bone marrow aspirations and biopsies (with hematology/oncology<br />
division)<br />
Trainees are expected to learn how to perform bone marrow aspirations and biopsies. They<br />
should aim to observe and then perform a total <strong>of</strong> about ten marrows. It is recognized that<br />
this goal may not be met. Some <strong>of</strong> this must be done while the trainee is on their UPMC-<br />
P/Hillman Cancer Center Hematology rotation. The remainder should be done later in their<br />
training (See below).<br />
A. The trainee will be able to observe and perform marrows as part <strong>of</strong> the clinical<br />
experience in hematology (see “Clinical Experience in Hematology”).<br />
B. The trainee is required to keep a record <strong>of</strong> each marrow observed and performed<br />
including the name <strong>of</strong> the patient, bone marrow number and diagnosis. For the bone<br />
marrows actually preformed, the person supervising the procedure needs to<br />
sign <strong>of</strong>f on the form. In addition, the aspirate smear and biopsy obtained must<br />
be reviewed by a faculty member to assess and document their adequacy. The<br />
form should be completed at the end <strong>of</strong> the rotation and given to Dr. Swerdlow’s<br />
coordinator in room PUH G333. There is also a hard copy <strong>of</strong> this form in the red<br />
packet.<br />
C. The trainee should also try to observe several pediatric marrows during the<br />
pediatric/laboratory hematology section <strong>of</strong> the rotation. These should also be<br />
recorded on the log when noting which are pediatric cases or use a separate sheet<br />
and check <strong>of</strong>f pediatric at the top.<br />
<strong>Resident</strong>s have the opportunity to perform bone marrow aspirates and biopsies at the VA hospital<br />
later in their training.
1.<br />
2.<br />
3.<br />
4.<br />
5.<br />
6.<br />
7.<br />
8.<br />
9.<br />
10.<br />
HEMATOPATHOLOGY ROTATION PERFORMANCE OF MARROW BIOPSIES AND ASPIRATES FORM<br />
Satisfactory completion <strong>of</strong> the rotation REQUIRES the return <strong>of</strong> this form.<br />
Name: Rotation Dates:<br />
AGE OF PATIENTS: ADULT PEDIATRIC<br />
BIOPSIES / ASPIRATES PERFORMED<br />
TO BE COMPLETED BY RESIDENT/FELLOW TO BE COMPLETED BY CLINICAL SUPERVISOR TO BE COMPLETED BY<br />
HEMATOPATHOLOGIST<br />
Patient Name PHB Number Aspirate Biopsy Signature<br />
Satisfactory<br />
Problem INITIALS Aspirate Biopsy<br />
(REQUIRED) (REQUIRED)<br />
S U NA S U NA<br />
(Please use back <strong>of</strong> form if needed.) S = SATISFACTORY U = UNSATISFACTORY (PUT COMMENT) NA = NOT<br />
APPLICABLE<br />
BIOPSIES / ASPIRATES OBSERVED<br />
Patient Name<br />
(REQUIRED)<br />
PHB Number<br />
(REQUIRED)<br />
Aspirate Biopsy Patient Name<br />
(REQUIRED)<br />
1. 7.<br />
2. 8.<br />
3. 9.<br />
4. 10.<br />
5. 11.<br />
6. 12.<br />
PHB Number<br />
(REQUIRED)<br />
Please get recut H& E and aspirate smear stained. Have attending hematopathologist review and evaluate in section above.<br />
Aspirate<br />
Biopsy<br />
TRAINEE SIGNATURE DATE DIRECTOR (OR DESIGNATE) SIGNATURE DATE<br />
PLEASE RETURN COMPLETED FORM PRIOR TO END OF ROTATION TO DR. SWERDLOW’S COODINATOR, RM G333.
Flow Cytometry Laboratory<br />
Experience
Flow Cytometry Laboratory Experience<br />
Introduction to flow cytometry<br />
Understanding <strong>of</strong> flow cytometric immunophenotypic techniques and interpretation <strong>of</strong><br />
the resultant data is an integral part <strong>of</strong> all the bone marrow and lymph node<br />
rotations. In addition, however there is a brief concentrated exposure to the flow<br />
cytometry laboratory including the technical aspects <strong>of</strong> flow cytometry and the basic<br />
operation <strong>of</strong> a flow cytometry laboratory. In most cases this will occur following the<br />
core pediatric bone marrow rotation (week 4). This is also the opportunity to learn<br />
about flow cytometric DNA content study.<br />
1. Meet with the Flow Cytometry Lab lead technologist or designate for orientation.<br />
2. Become familiar with instrumentation and procedures in laboratory under the guidance<br />
<strong>of</strong> the lead technologist.<br />
• Immunostaining.<br />
• Acquisition and analysis using flow cytometry.<br />
• Quality control/quality assurance.<br />
• Operation <strong>of</strong> a large clinical flow cytometry laboratory<br />
3. Complete checklist.<br />
4. Educational materials available:<br />
• Flow Cytometry First Principles. Alice L. Givan.<br />
• Flow Cytometry in Clinical Diagnosis. 4 rd edition, editors: D. Keren, J.P.<br />
McCoy and J.L. Carey.<br />
• Flow cytometric immunophenotyping for hematologic neoplasms. Blood<br />
111(8)3941-3967, 2008.
Flow Cytometry Rotation Checklist<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Orientation with the Lead Technologist or her designate.<br />
Understand the principles <strong>of</strong> flow cytometric immunophenotyping.<br />
Gain familiarity with instrument set –up, compensation and quality<br />
control.<br />
Gain familiarity with procedures for surface and cytoplasmic antibody<br />
staining.<br />
Understand the principles <strong>of</strong> back-gating and gating using light<br />
scatter and antigen expression<br />
Understand the procedure for DNA / ploidy analysis<br />
Perform additional data analysis using DIVA s<strong>of</strong>tware on specimens<br />
previously analyzed in the clinical Flow Cytometry Laboratory<br />
Know the immunophenotypic characteristics <strong>of</strong> the following entities:<br />
Observed Observed<br />
Read<br />
Actual Teaching<br />
Diagnosis/Finding<br />
About<br />
Case File Case<br />
Normal bone marrow<br />
<br />
Hematogones<br />
<br />
Reactive Lymph Node<br />
<br />
Lymphoma<br />
Follicular lymphoma<br />
Chronic lymphocytic leukemia/Small lymphocytic lymphoma<br />
Mantle Cell lymphoma<br />
MALT lymphoma<br />
Diagnosis/Finding<br />
Burkitt lymphoma<br />
T-cell lymphoma<br />
Observed<br />
Actual<br />
Case<br />
<br />
<br />
<br />
<br />
Observed<br />
Teaching<br />
File Case<br />
Read<br />
About
Chronic leukemia<br />
Hairy Cell Leukemia<br />
Prolymphocytic Leukemia, B-cell phenotype<br />
Prolymphocytic Leukemia, T-cell phenotype<br />
Sézary Syndrome<br />
Large Granular Lymphocytic Leukemia<br />
Adult T-cell Leukemia / Lymphoma<br />
Acute Myeloid Leukemia<br />
Acute Promyelocytic Leukemia<br />
Acute Myeloid Leukemia associated with t(8;21)<br />
Acute Myeloid Leukemia associated with inv(16)<br />
Acute Myeloid Leukemia with monocytic differentiation<br />
Acute Megakaryocytic Leukemia<br />
Acute Lymphoblastic Leukemia<br />
Precursor B-cell<br />
Precursor T-cell<br />
Minimal Residual Acute Lymphoblastic Leukemia<br />
Paroxysmal Nocturnal Hemoglobinuria<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Chronic Granulomatous Disease<br />
Neutrophil Oxidative Burst Analysis<br />
<br />
I have completed the Flow Cytometry Checklist.<br />
Name __________________________________________ (printed)<br />
Date<br />
__________________________________________ (signature)<br />
__________________________________________
Flow Cytometry Panels, Available Antibodies, Guidelines,<br />
Test Specifications and Templates<br />
Lymphoproliferative Panel (PB or BM)<br />
FITC PE PerCP-Cy5.5 PE-Cy7 APC APC-H7 V450 V500<br />
CD14 CD13&33 CD45 CD34<br />
CD16&57 CD7 CD4 CD3 CD56 CD8 CD2 CD45<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
*cTdT cMPO cCD3 cCD34<br />
*add to Pediatric specimens; “c” = cytoplasmic<br />
Lymphoproliferative Panel (LN)<br />
FITC PE PerCP-Cy5.5 PE-Cy7 APC APC-H7 V450 V500<br />
CD16&57 CD7 CD4 CD5 CD56 CD3 CD2 CD8<br />
Kappa Lambda CD5 CD19 CD10 CD14 CD20 CD45<br />
**cTdT MPO CD3 CD34<br />
*as needed<br />
**add to Pediatric specimens; “c” = cytoplasmic<br />
8 Color CSF Tube<br />
FITC PE PerCP- PE-Cy7 APC APC-H7 V450 V500<br />
Cy5.5<br />
Kappa Lambda CD5 CD13/33 CD34 CD14 CD19 CD45
MDS / Cytopenia Panel (BM and PB)<br />
FITC PE PerCP-Cy5.5 PE-Cy7 APC APC-H7 V450 V500<br />
CD36 CD123 CD64 CD33 CD34 CD14 HLA- CD45<br />
DR<br />
CD15 CD56 CD117 CD13 CD11b CD16 HLA- CD45<br />
DR<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
CD16&57 CD7 CD4 CD3 CD56 CD8 CD2 CD45<br />
CD7 CD13&33 CD19 CD56<br />
Acute Leukemia Panel (PB and BM)<br />
FITC PE PerCP-Cy5.5 PE-Cy7 APC APC-H7 V450 V500<br />
CD36 CD123 CD64 CD33 CD34 CD14 HLA- CD45<br />
DR<br />
CD15 CD56 CD117 CD13 CD11b CD16 HLA- CD45<br />
DR<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
CD16&57 CD7 CD4 CD3 CD56 CD8 CD2 CD45<br />
CD7 CD13&33 CD19 CD56<br />
cTdT cMPO cCD3 cCD34<br />
*CD58 CD123 CD34 CD19 CD10 CD38 CD20 CD45<br />
*add to Pediatric specimens; “c” = cytoplasmic<br />
Myeloma Panel<br />
FITC PE PerCP- PE-Cy7 APC APC-H7 V450 V500<br />
Cy5.5<br />
CD14 CD13&33 CD45 CD34<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
CD16&57 CD7 CD4 CD3 CD56 CD8 CD2 CD45<br />
CD3 CD138 CD19 CD56<br />
cLambda CD138 CD19 cKappa<br />
“c” = cytoplasmic
CLL Panel (PB or BM)<br />
FITC PE PerCP- PE-Cy7 APC APC-H7 V450 V500<br />
Cy5.5<br />
CD14 CD13&33 CD45 CD34<br />
CD16&57 CD7 CD4 CD3 CD56 CD8 CD2 CD45<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
FMC7 CD23 CD19 CD5<br />
cZAP70 CD19 CD3 CD5<br />
“c” = cytoplasmic<br />
Mini (B) Acute Lymphoblastic Leukemia (PB or BM)<br />
Previous diagnosis <strong>of</strong> B-lineage acute lymphoblastic leukemia (ALL) established at UPMC-<br />
Presbyterian/Shadyside including flow cytometric evaluation (NOT flow only). PB or BM<br />
submitted for evaluation <strong>of</strong> ALL. Review previous phenotype for additional aberrant<br />
markers.<br />
FITC PE PerCP-Cy5.5 PE-Cy7 APC APC-Cy7 V450 V500<br />
CD14 CD13&33 CD45 CD34<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
CD58 CD123 CD34 CD19 CD10 CD38 CD20 CD45<br />
cTdT cMPO cCD3 cCD34<br />
”c”= cytoplasmic
Mini (T) Acute Lymphoblastic Leukemia (PB or BM)<br />
Previous diagnosis <strong>of</strong> T-lineage acute lymphoblastic leukemia (ALL) established at UPMC-<br />
Presbyterian/Shadyside including flow cytometric evaluation (NOT flow only). PB or BM<br />
submitted for evaluation <strong>of</strong> ALL. Review previous phenotype for additional aberrant<br />
markers.<br />
FITC PE PerCP- PE-Cy7 APC APC-<br />
Cy5.5<br />
Cy7<br />
CD14 CD13&33 CD45 CD34<br />
V450<br />
V500<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
CD16&57 CD7 CD4 CD3 CD56 CD8 CD2 CD45<br />
CD7 CD13&33 CD5 CD56<br />
cTdT cMPO cCD3 cCD34<br />
”c”= cytoplasmic<br />
Mini (AML) Leukemia (PB or BM)<br />
Previous diagnosis <strong>of</strong> acute myeloid leukemia (AML) established at UPMC-<br />
Presbyterian/Shadyside including flow cytometric evaluation (NOT flow only). PB or BM<br />
submitted for evaluation <strong>of</strong> AML. Review previous phenotype for additional aberrant<br />
markers.<br />
FITC PE PerCP- PE-Cy7 APC APC-H7 V450 V500<br />
Cy5.5<br />
CD36 CD123 CD64 CD33 CD34 CD14 HLA- CD45<br />
DR<br />
CD15 CD56 CD117 CD13 CD11b CD16 HLA- CD45<br />
DR<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
Basic PB or BM Screen<br />
Limited evaluation <strong>of</strong> lymphoid and myeloid cells to be used when the following criteria are<br />
met: no significant history, no cytopenias, and no morphologic abnormality e.g. staging for<br />
Hodgkin lymphoma. Also, to follow-up documented CML, or rule-out a myeloproliferative<br />
disorder such as CML, polycythemia vera (PV), essential thrombocythemia (ET). or idiopathic<br />
myel<strong>of</strong>ibrosis.<br />
FITC PE PerCP- PE-Cy7 APC APC-H7 V450 V500<br />
Cy5.5<br />
CD14 CD13&33 CD45 CD34<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45
Mini B-cell Lymphoma Follow-up (PB or BM)<br />
Limited evaluation for follow-up <strong>of</strong> B-cell lymphoma with previous diagnosis at UPMC-<br />
Presbyterian/Shadyside including flow cytometric evaluation (NOT flow only). PB or BM<br />
submitted for evaluation <strong>of</strong> lymphoma, and morphology review is consistent with that<br />
diagnosis and does NOT demonstrate another disease process e.g. increased blasts.<br />
FITC PE PerCP- PE-Cy7 APC APC-H7 V450 V500<br />
Cy5.5<br />
CD14 CD13&33 CD45 CD34<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
bcl‐2 testing should be performed on all cases with a history <strong>of</strong> follicular lymphoma or if the<br />
requisition asks for testing for bcl‐2, bcl‐2 gene rearrangement, or t(14:18) testing (even if this is<br />
indicated in the molecular or cytogenetic area <strong>of</strong> the requisition).<br />
Mini CLL Follow-up (PB or BM)<br />
Limited evaluation for follow-up <strong>of</strong> chronic lymphocytic leukemia (CLL) or small lymphocytic<br />
lymphoma (SLL) with previous diagnosis at UPMC-Presbyterian/Shadyside including flow<br />
cytometric evaluation. PB or BM submitted for evaluation <strong>of</strong> CLL, and morphology review is<br />
consistent with that diagnosis and does NOT demonstrate another disease process e.g.<br />
increased blasts.<br />
FITC PE PerCP- PE-Cy7 APC APC-H7 V450 V500<br />
Cy5.5<br />
CD14 CD13&33 CD45 CD34<br />
FMC-7 CD23 CD19 CD5<br />
CD38 CD19 CD5<br />
Kappa Lambda CD5 CD19 CD10 CD38 CD20 CD45<br />
cZAP-70 CD19 CD3 CD5<br />
”c”= cytoplasmic
Validated Extra Tubes:<br />
FITC PE PerCP- PE-Cy7 APC APC-H7 V450 V500<br />
Cy5.5<br />
CD52*<br />
bcl-2 CD10 CD20<br />
bcl-2 CD10 CD19<br />
CD7 CD26 CD3 CD4<br />
TCR a/b TCR g/d CD3 CD25<br />
CD57 CD8 CD3<br />
CD3 CD7 CD4<br />
FMC-7 CD23 CD19 CD5<br />
CD38 CD19 CD5<br />
ZAP-70 CD19 CD3 CD5<br />
CD103 CD11c CD20 CD25<br />
Blkd.K Blkd.L CD20 CD10<br />
Blkd.K Blkd.L CD19 CD5<br />
CD61 CD13/33 CD41a CD34<br />
Tdt CD22 CD79a CD34 CD45<br />
<br />
<br />
CD52 FITC for consideration for CAMPATH therapy put in combination with previously<br />
tested antibodies<br />
CD123-PE for consideration in new acute leukemias or plasmacytoid<br />
dendritic cell neoplasms
ANTIBODIES AVAILABLE IN THE FLOW CYTOMETRY LABORATORY<br />
FITC PE PerCP PerCP-<br />
CY5.5<br />
PE-<br />
Cy7<br />
APC<br />
APC-<br />
H7<br />
CD1a<br />
X<br />
CD2 X X X X<br />
CD3 X X X X X X X<br />
CD4 X X X<br />
CD5 X X X X<br />
CD7 X X*<br />
CD8 X X X X X<br />
CD10 X X X<br />
CD11b X X<br />
CD11c<br />
X<br />
CD13 X X<br />
CD14 X X X*<br />
CD15 X X<br />
CD16 X* X X<br />
CD18 X<br />
CD19 X X X* X<br />
CD20 X X X X X X<br />
CD22 X X X*<br />
CD23<br />
X<br />
CD24<br />
X<br />
CD25<br />
X<br />
CD26<br />
X<br />
CD33 X X X<br />
CD34 X X X<br />
CD38 X X<br />
CD41A<br />
X<br />
CD43 X<br />
CD45 X X X* X<br />
CD45RA X<br />
CD45RO<br />
X<br />
CD52 X<br />
CD55<br />
CD56 X X<br />
CD57 X*<br />
CD59* X*<br />
CD61 X<br />
CD62L<br />
X<br />
CD79b<br />
X<br />
CD81 X<br />
CD103 X<br />
CD117<br />
X<br />
CD123<br />
X<br />
CD138<br />
X<br />
A/B X<br />
G/D<br />
X<br />
Glyco A X*<br />
(235a)*<br />
BCL2 X<br />
V450 V500 Simulset
FITC PE PerCP PerCP-<br />
CY5.5<br />
X<br />
PE-<br />
Cy7<br />
APC<br />
APC-<br />
H7<br />
FMC7<br />
HLA-DR X* X<br />
MPO<br />
X<br />
TDT X<br />
Zap 70 X<br />
Lambda X X<br />
Kappa X X X<br />
Im Tech<br />
G1<br />
BD G1 X X<br />
BD G2a X X X<br />
V450 V500 Simulset<br />
Sim K/L<br />
Sim 45/14<br />
Sim<br />
G1/G1<br />
X<br />
X<br />
X
Guidelines for Flow Cytometry Staining Decisions<br />
1. Pathologists Decisions. The pathologist who is assigned to the appropriate service<br />
should be called for a decision in any <strong>of</strong> the following circumstances:<br />
a. Limited specimen. Either there are too few cells to set-up the indicated panel or the<br />
appropriate panel would use the specimen in its entirety. For some limited CSF<br />
specimens technologists can make decision if there is a previous history, with flow<br />
cytometry run in our system, <strong>of</strong> precursor-B acute lymphoblastic leukemia, acute<br />
myeloid leukemia, CD10 positive B-cell lymphoma, CD5 positive B-cell lymphoid<br />
neoplasm. The Pathologist should be called for limited CSF samples if there is no<br />
history or history <strong>of</strong> another malignancy.<br />
b. There are questions about any <strong>of</strong> the information available (including the history,<br />
clinical information, test requested or morphologic appearance).<br />
c. For whatever reason, there is uncertainty regarding the appropriate panel.<br />
d. A technologist who has met the required morphology or decision competency level is<br />
not available.<br />
The following protocol is recommended when calling a pathologist for a decision:<br />
a. Identify yourself and inform the pathologist that you are calling for a flow decision.<br />
b. Notify the pathologist <strong>of</strong> the following information:<br />
i. Cellularity / adequacy <strong>of</strong> specimen. Alert the pathologist if the cellularity is low.<br />
Tell them the total number <strong>of</strong> cells and / or approximately how many tubes can<br />
be set-up.<br />
ii. Specimen Type (peripheral blood, bone marrow, fluid etc.).<br />
iii. Flow-only or In-house.<br />
iv. Test requested (evaluation <strong>of</strong> lymphocytes or blasts).<br />
v. Clinical Information provided.<br />
vi. Previous pertinent diagnoses in CoPath including the phenotype.<br />
vii. Presence and quantity <strong>of</strong> abnormal cells including blasts / immature<br />
mononuclear cells, lymphocytes / abnormal lymphocytes, plasma cells, and<br />
unidentifiable cells.<br />
2. Technologist Decisions. Flow decisions can be made by a technologist who has met<br />
the required morphology and decision competency level, in the following circumstances:<br />
a. New Adult Acute Leukemia. A complete “Acute Leukemia Panel” can be set-up if<br />
the smear demonstrates numerous blasts, immature cells, or monocytes. The<br />
designated hematopathologist should be paged with a text message to alert them<br />
about a possible new case <strong>of</strong> acute leukemia. The pathologist should be called<br />
directly if there are any questions about the tubes that should be set-up, the urgency
<strong>of</strong> the results, or the morphologic appearance. The pathologist should also receive a<br />
text message when the completed case is being tubed.<br />
b. Full panel. One <strong>of</strong> the standard complete panels can be set-up if the requisition<br />
indicates the disease entity being evaluated, and both the previous history in CoPath,<br />
and the morphologic review are consistent with that diagnosis. Technologists are<br />
encouraged to be proactive in ordering any additional tubes indicated by previous flow<br />
cytometric results or the information provided on the requisition e.g. the bcl-2 extra in<br />
the evaluation <strong>of</strong> BM involvement by follicular lymphoma. Novel, untested,<br />
combinations are NOT recommended unless the antibodies have already been<br />
evaluated in their standard combinations.<br />
c. Limited panel. One <strong>of</strong> the authorized limited panels (see attached list) can be<br />
ordered if the following criteria are met:<br />
i. The patient is being evaluated for a disease entity for which there is a<br />
previous diagnosis at UPMC-Presbyterian/Shadyside including flow<br />
cytometric evaluation (NOT flow only, except for PB evaluation <strong>of</strong> CLL).<br />
ii.<br />
The requisition indicates follow-up <strong>of</strong> that disease entity.<br />
iii.<br />
The morphologic findings are consistent with that diagnosis,<br />
or treatment <strong>of</strong> that entity, and do NOT indicate another disease<br />
process.<br />
d. CSF specimens. If there are only enough cells available for one tube, flow<br />
cytometry technologists can make a decision <strong>of</strong> which tube to run, based on<br />
the following guidelines:<br />
i. Previous history <strong>of</strong> precursor-B acute lymphoblastic leukemia with flow cytometry<br />
run in our system:<br />
• CD10 positive - set up CD10 / CD13&CD33 / CD19 / CD34<br />
• CD10 negative - call for decision<br />
• Any questions - call for decision<br />
ii. Previous history <strong>of</strong> acute myeloid leukemia with flow cytometry run in our system:<br />
• CD34 positive - Set up 8-color CSF tube<br />
• CD34 negative - call for decision<br />
• Any questions - call for decision<br />
iii. Previous history <strong>of</strong> B-cell lymphoma with flow cytometry or previous diagnostic<br />
evaluation in our system:<br />
i. set up 8-color B-cell tube<br />
iv. No history <strong>of</strong> a hematolymphoid malignancy, such as leukemia, lymphoma,<br />
myeloma, after review <strong>of</strong> CoPath and available clinical information:<br />
i. set up 8-color CSF tube (K/L/CD5/CD13&33/CD34/CD14/CD19/CD45).<br />
3. Technologist Ordering <strong>of</strong> Extra’s. After review <strong>of</strong> the results from the original panel,<br />
technologists are encouraged to be proactive in ordering any additional tubes indicated,<br />
or text paging the Pathologists to alert them <strong>of</strong> the possible need for Extra’s. For<br />
example:
a. bcl-2 extra: abnormal CD10 positive B-cell population<br />
b. FMC-7 / CD23 / CD19 / CD5: abnormal CD5 positive B-cell population.<br />
c. CD103 / CD11c / CD20 / CD25: abnormal CD10 negative, CD5 negative B-cell<br />
population.
TEST SPECIFICATIONS: CLINICAL FLOW CYTOMETRY LABORATORY<br />
Leukemia Panels, CLL/Lymphoma Panels, T-Lymphocyte Subset Panels, PNH, NOBA<br />
DELIVER ALL SAMPLES DIRECTLY TO THE FLOW CYTOMETRY LABORATORY<br />
Scaife Hall<br />
Room S-763 (7 th floor)<br />
3550 Terrace Street<br />
Pittsburgh, PA 15261<br />
(412) 624-3746<br />
FLOW CYTOMETRY LAB HOURS OF OPERATION<br />
Monday through Friday: 8:00 am to 6:00 pm. In all cases, notify the lab before the specimen is sent<br />
(412- 624-3746). It is recommended that on Friday specimens be received by 5 pm.<br />
Saturday: Emergency specimens can be processed on Saturday. The laboratory must be notified by 12<br />
pm and the specimen must arrive by 1 pm. The hematopathologist on call can be reached through the<br />
UPMC Oakland operator at (412) 647-2345.<br />
Sundays and Holidays: The lab is closed on Sunday and the following holidays: New Year’s Day, Martin<br />
Luther King Day, Memorial Day, July 4, Labor Day, Thanksgiving Day and Christmas. Specimens should<br />
be received by 3 pm on the day before a holiday.<br />
SPECIAL INSTRUCTIONS<br />
<br />
<br />
<br />
Send a completed requisition. In addition FAX the requisition to 412-624-6863 in advance <strong>of</strong><br />
sending the specimen.<br />
See specific instructions for storage/transport <strong>of</strong> specimens.<br />
Use adequate safety measures in transporting specimens.<br />
LEUKEMIA, CLL/LYMPHOMA PANELS<br />
Test Description<br />
These tests utilize a panel <strong>of</strong> monoclonal antibodies in the immunophenotypic analysis <strong>of</strong> hematopoietic<br />
and lymphoid proliferation. The panels are used to assess cell lineage and to look for features that<br />
support a neoplastic rather than reactive proliferation.<br />
Specimen Requirements<br />
Bone marrow aspirate: Minimum <strong>of</strong> 2 ml in a heparinized (green top) tube. Store/transport<br />
specimen at room temperature. If possible send one non-heparinized,<br />
unstained aspirate smear.<br />
Peripheral blood:<br />
One EDTA (preferred; purple top) tube or heparinized (green top) tube<br />
with at least 5 ml <strong>of</strong> blood. Store/transport specimen at room<br />
temperature.<br />
Lymph nodes:<br />
Preferably 1 cm 3 fresh tissue in RPMI or any other growth media.<br />
Normal saline may be used if RPMI is unavailable and transport time is<br />
kept to a minimum. A small portion <strong>of</strong> all tissues (or other tissues) will be<br />
processed for histologic sections. Send tissue as soon as possible –<br />
preferably to be received within 24 hours. Store specimen at<br />
2-8 o C if delay in transport.
Body fluids: Preferably should be a minimum <strong>of</strong> approximately 100,000 cells (e.g. 100<br />
cells/ul x 1 ml; 10 cells/ul x 10 ml). Testing can be attempted even on<br />
very low count specimens. Send specimen as soon as possible.<br />
Store at 2-8 o C if delay in transport.<br />
Fine needle aspirates: Place cores (preferably two) and any additional aspirate in RPMI or other<br />
growth media. Normal saline may be used if RPMI is unavailable and<br />
transport time is kept to a minimum. Send tissue as soon as possible –<br />
preferably to be received within 24 hours. Store specimen at 2-8 o C<br />
if delay in transport.<br />
T-LYMPHOCYTE SUBSET EVALUATION<br />
Test Description<br />
This test may be identified as T Cell Subsets, T and B Cells, CD4:CD8 counts and other synonyms. The<br />
test panel includes a Total T or Pan T antibody, a Total B cell or Pan B antibody, a Total Helper antibody,<br />
a Total Suppressor antibody, the Helper/Suppressor, and a Total Natural Killer antibody. There are no<br />
functional tests associated with these antibodies.<br />
A limited CD4 evaluation can be performed if requested.<br />
Specimen Requirements<br />
One 4 ml EDTA (purple top) tube or two BD microtainer (purple top) pediatric tubes. Store/transport<br />
specimen at room temperature. Testing must performed within 48 hours <strong>of</strong> specimen collection.<br />
EVALUATION FOR PAROXYSMAL NOCTURNAL HEMOGLOBINURIA (PNH)<br />
Test Description<br />
The flow cytometric test for PNH evaluates the GPI-linked markers: CD59 on RBCs; CD24 and CD16 on<br />
Neutrophils and CD14 on Monocytes. The WBC assay also determines binding <strong>of</strong> the fluorochrome<br />
labeled toxin Aerolysin.<br />
Specimen Requirements<br />
One 4 ml EDTA (purple top) tube. Store/transport specimen at room temperature. Testing must be<br />
performed within 48 hours <strong>of</strong> specimen collection.<br />
EVALUATION FOR NEUTROPHIL OXIDATIVE BURST ASSAY (NOBA)<br />
Test Description<br />
This test is a replacement for the Nitro Blue Tetrazolium (NBT) test for Chronic Granulomatous Disease<br />
(CGD). The neutrophil oxidative burst assay measures the respiratory burst <strong>of</strong> neutrophils following<br />
stimulation with phorbol 12-myristate 13-acetate (PMA). Patients with CGD lack the usual oxidative burst.<br />
Specimen Requirements<br />
One 4 ml EDTA (purple top) tube kept at room temperature. Store/transport specimen at room<br />
temperature. Testing must be performed within 24 hours <strong>of</strong> specimen collection. In addition a normal<br />
control (from an UNRELATED donor) MUST accompany the specimen.<br />
CONTACTS, DIVISION OF HEMATOPATHOLOGY<br />
Steven H. Swerdlow, M.D. Director, Division <strong>of</strong> <strong>Hematopathology</strong> (412) 647-5191<br />
Raymond Felgar, M.D. Co-Director, Flow Cytometry Lab (412) 647-8780<br />
Christine Roth, M.D. Co-Director, Flow Cytometry Lab (412) 692-2090<br />
Karen Freilino Lead Technologist, Flow Cytometry (412) 624-3746<br />
Rev 4/13
ICD9 Codes for Flow<br />
A list <strong>of</strong> ICD9 Codes commonly received in the Flow Laboratory. This list is to be used as an<br />
aid in interpreting ICD9 codes. It is not to be used to assign ICD9 codes to a case.<br />
IDC9<br />
Disease<br />
78.9 (V78.9) Blood Screen (NOS)<br />
79.99 Viral Infection NOS<br />
172.6 Malignant Melanoma<br />
194.9 Malignant Endocrine Neoplasm (NOS)<br />
201.9 Hodgkin’s Disease (NOS)<br />
202.1 Mycosis Fungoides Unspec Site<br />
202.4 Hairy Cell Leukemia Unspec Site<br />
202.6 Mastocytosis<br />
202.8 Lymphoma Unspec Site<br />
203 Multiple Myeloma No Remission<br />
203.01 Multiple Myeloma In Remission<br />
204.1 CLL - No remission<br />
205 Myeloid leukemia<br />
205.1 CML - No remission<br />
205.11 CML - In remission<br />
207.8 Mast cell leukemia<br />
208.9 Unspecified Leukemia<br />
238.4 Polycythemia Vera<br />
238.7 Lymphoproliferative Chronic (NOS)<br />
238.7 Myeloproliferative Chronic (NOS)<br />
273.3 Macroglobulinemia<br />
284.8 Aplastic Anemia NEC<br />
284.9 Aplastic Anemia NOS<br />
285.6 Lymphadenopathy<br />
285.9 Anemia (NOS)<br />
287.4 Secondary Thrombocytopenia<br />
288 Agranulocytosis<br />
288.1 Function Disorder Neutrophils<br />
288.8 White Blood Disease NEC<br />
289.89 Myel<strong>of</strong>ibrosis<br />
709.9 Skin Disorder NOS<br />
786.5 Chest Pain<br />
789.2 Splenomegaly
Adult Bone Marrow<br />
Experience
Adult Bone Marrow Experience (~4 weeks)<br />
1. Adult Bone Marrow Sign Out<br />
A. Sessions with Adult Bone Marrow Technologists -- if not already done during<br />
pediatric marrow rotation (eg AP only residents), please set up a time to review<br />
peripheral blood and bone marrow aspirate smears with technologists during<br />
your first week.(typically Tuesday afternoon is best)<br />
B. Participation in sign-out with staff. Inform Hematopathologist on BM #1 Service that<br />
you are starting the rotation and review expectations in terms <strong>of</strong> bone marrow<br />
preview, sign-out, dictation, and pro<strong>of</strong>ing ( see “Trainee Responsibilities during<br />
Bone Marrow Rotation”).The trainee should discuss with the faculty the optimal<br />
number <strong>of</strong> cases to evaluate prior to sign-out. .The number <strong>of</strong> cases the resident<br />
is expected to evaluate will increase progressively during the rotation.<br />
C. Review results <strong>of</strong> ancillary procedures performed on marrows you have reviewed<br />
and read addenda that faculty issue (see Special Procedure Review by<br />
<strong>Resident</strong>/<strong>Fellow</strong> in CoPath).<br />
D. Review <strong>of</strong> educational materials.<br />
<br />
<br />
<br />
Blood Cell Morphology. (Published by the ASCP). If not already done during<br />
pediatric marrow rotation (ie AP only residents).. review the RBC, WBC, Normal<br />
and Abnormals Binders with CDs in <strong>Hematopathology</strong> library (G323) or images<br />
and PDF key are also on shared resident drive, under “CP Lecture<br />
Material\Hemepath\ASCP Hematology Images”<br />
A teaching set <strong>of</strong> peripheral blood and body fluid smears is available for checkout<br />
from the medical secretary (G306)<br />
A set <strong>of</strong> cytochemical stains and pb/bm smears is available from the bone<br />
marrow technologists. Individual faculty members also have teaching slides.<br />
Foucar, K. Bone Marrow Pathology, 2 nd Edition, ASCP Press, 2001.<br />
<br />
<br />
<br />
<br />
<br />
<br />
Foucar, K, Viswanatha DS, Wilson S (Eds). Non-neoplastic disorders <strong>of</strong> bone<br />
marrow. Atlas <strong>of</strong> non-tumor pathology. AFIP, 2008.<br />
Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E., Pileri, S.A., Stein, H., Thiele,<br />
J., Vardiman, J. (Eds.): WHO Classification <strong>of</strong> Tumours Pathology <strong>of</strong><br />
Haematopoietic and Lymphoid Tumours, IARC, Lyon, 2008.<br />
Keren DF, McCoy JP Jr, Carey JL (Eds.): Flow Cytometry in Clinical Diagnosis,<br />
4 rd Edition, ASCP, 2007.<br />
Bain, BJ, Clark, DM, Lampert, IA, Wilkins, BS. Bone Marrow Pathology, 3 rd<br />
Edition, Blackwell Science, 2001.<br />
Kjeldsberg CR: Practical Diagnosis <strong>of</strong> Hematologic Disorders, 4 th Edition, ASCP,<br />
2006.<br />
Leonard, D Diagnostic Molecular Pathology (Volume 41 in the series “Major<br />
Problems in Pathology”), Saunders, 2003.
Stramatoyannopoulos M, Perlmutter V. Molecular Basis <strong>of</strong> Blood Diseases, 3 rd<br />
Edition, W.B. Saunders, 2001.<br />
Additional Resources:<br />
Knowles DM. Neoplastic <strong>Hematopathology</strong>, 2 nd Edition, Lippincott Williams &<br />
Wilkins, 2000.<br />
<br />
Peterson LC and Brunning RD. Chapter 37. Bone Marrow specimen<br />
Processing, pp1391-1406.<br />
Li C-Y and Yam LT. Chapter 38. Cytochemical, Histochemical and<br />
Immunohistochemical Analysis <strong>of</strong> the Bone Marrow<br />
See list <strong>of</strong> web-based resources at end <strong>of</strong> manual.<br />
NOTE: Reading is an important component <strong>of</strong> this rotation. It is recognized that not all<br />
<strong>of</strong> the above resources can be used nor can most be read in entirety. Use <strong>of</strong> electronic<br />
and other resources to find and read up-to-date journal articles is also critical.<br />
General Trainee Responsibilities during Bone Marrow Rotation<br />
Adequate preparation <strong>of</strong> a bone marrow prior to sign out includes:<br />
1. Aspirate smears are usually available the day before the biopsy is processed (i.e. the<br />
day before sign-out). Ideally cases should be scanned the day before sign-out in order<br />
to select appropriate cases in which trainees will be expected to assume a greater<br />
degree <strong>of</strong> responsibility – please coordinate with your attending. On these cases, please<br />
do your own differential counts to see how they compare with the technologists’ counts<br />
on the next day. Particularly once you have some experience, the differentials should be<br />
on the more interesting/difficult cases. Contact physician if necessary (e.g., if after<br />
review with staff pathologists, a new acute leukemia is seen). Trainees will have<br />
increasing responsibility for independent preview, as they are more experienced.<br />
2. Obtain sufficient history from computer, chart or physician’s <strong>of</strong>fice to understand reason<br />
for marrow being performed and to understand any coexistent disease process, drug<br />
usage, exogenous toxins, etc. which might affect bone marrow interpretation. Look up<br />
any appropriate laboratory data such as folate, B12, and iron (plus TIBC) in anemias or<br />
macrocytosis, results <strong>of</strong> SPEP, UPEP, and immun<strong>of</strong>ixation in evaluation <strong>of</strong> plasma cell<br />
disorders. If possible, this should be obtained prior to initial review with staff pathologist.<br />
The technologist’s history may or may not be sufficient.<br />
3. Preview <strong>of</strong> peripheral blood smear, marrow aspirate smears and biopsies on all<br />
assigned cases.<br />
4. Gather and interpret any ancillary studies which have been performed such as flow<br />
cytometry.<br />
5. Review prior marrow and surgical pathology specimens where appropriate (e.g.<br />
leukemic marrow case where looking for residual/recurrent disease, extent <strong>of</strong> prior<br />
disease in follow-up <strong>of</strong> plasma cell neoplasia).<br />
6. Write down description and diagnosis in pencil on template or indicate agreement or<br />
disagreement with technologist’s comments with a check mark or other notation.<br />
.Discuss the format <strong>of</strong> bone marrow report with the staff pathologist on service.
7. In consultation with staff pathologist, dictate reports either upon completion <strong>of</strong> sign-out or<br />
after each case. Please use whatever method will get the cases out ASAP. See specific<br />
guidelines on next page.<br />
8. The staff pathologist may ask you to pro<strong>of</strong> and correct the reports you have dictated. If<br />
you will be doing this, ask the secretary not to send the report to the pathologist’s queue<br />
(i.e. do not “final” the report). Trainee should final the report once it is pro<strong>of</strong>ed.<br />
9. With increasing experience, fellows will have a more independent role in bone marrow<br />
evaluations with increasing responsibility as designated by faculty.<br />
10. Bone marrow report:<br />
Peripheral blood smear should focus on morphologic features <strong>of</strong> (1) red blood<br />
cells, (2) leukocytes and (3) platelets. Any abnormal or atypical features<br />
should be described in detail.<br />
<br />
Morphologic description <strong>of</strong> bone marrow should address the following<br />
features:<br />
‣ Cellularity<br />
‣ Presence <strong>of</strong> abnormal infiltrates (lymphoid aggregates, clusters <strong>of</strong><br />
immature myeloid cells, granuloma, metastatic tumor infiltrates, etc.)<br />
‣ Myeloid-to-erythroid ratio<br />
‣ Maturation <strong>of</strong> megakaryocytic, erythroid and granulocytic precursors<br />
‣ Presence (and severity) <strong>of</strong> dysplasia<br />
‣ Bone trabeculae
Specific Guidelines for <strong>Resident</strong>s and <strong>Fellow</strong>s on Bone Marrow Service<br />
.<br />
I. Previewing<br />
a. Urgent cases that faculty should be informed about without delay:<br />
i. New acute leukemias.<br />
ii. Day 14 status post chemotherapy induction for acute myeloid leukemia.<br />
b. Please designate number <strong>of</strong> cells to be counted; generally 500 cell count if there<br />
is any suspicion for a myeloid neoplasm, 300 cell count for lymphoma/myeloma<br />
staging cases.<br />
c. An iron stain should be ordered if there is anemia, microcytosis, macrocytosis, or<br />
elevated RDW.<br />
i. Please order it on the aspirate smears unless there are no spicules.<br />
ii. If there are no spicules, order it on the touch imprint or core biopsy.<br />
iii. Exception: An iron stain is likely not needed for anemia or abnormal indices<br />
during induction or consolidation for acute leukemia.<br />
II.<br />
III.<br />
Preparation prior to sign-out<br />
a. When applicable, have the most recent bone marrow report and diagnostic<br />
bone marrow report printed out.<br />
b. If available, diagnostic and most recent bone marrow slides should be<br />
pulled out and reviewed and reports printed<br />
c. When applicable, review the prior diagnosis, pertinent immunophenotype and<br />
cytogenetics.<br />
d. Preview the case, including the flow cytometry.<br />
i. Arrange a time to sign-out with a faculty member so that you will have<br />
enough time for previewing, which is very important in resident education.<br />
ii. Be ready to discuss the case and ask questions.<br />
Immunohistochemistry and other studies<br />
a. Order 5-10 blanks to be cut whenever immunohistochemistry studies are<br />
ordered on our bone marrow cases.<br />
b. Order stains through the bone marrow technologists (Audix stain line 412-802-<br />
3273)<br />
c. Please use the table for dictating the results <strong>of</strong> the immunohistochemistry<br />
studies.<br />
i. Transcriptions know how to format the results in the table<br />
ii. Quick text = “HISTRPT.”<br />
d. Please remember to justify why immunohistochemistry has been performed if<br />
flow has also been performed.<br />
Quick text choices: FLOW/IHC1 or FLOW/IHC2.
IV.<br />
Quality assurance is very important<br />
a. Assess the adequacy <strong>of</strong> aspirate smears and core biopsy: Adequate,<br />
suboptimal, or inadequate, using the following criteria below:<br />
Bone Marrow Adequacy Criteria:<br />
Biopsy (trephine):<br />
Adequate (A) 15 mm gross length, at least 10 partially preserved intertrabecular<br />
areas (40x)<br />
Suboptimal (S) less than 15 mm length, at least 5 partially preserved<br />
intertrabecular areas<br />
Inadequate (I) less than 5 mm, fewer than 5 partially preserved intertrabecular<br />
areas<br />
Particle/clot:<br />
Adequate (A) at least 10 partially preserved marrow particles/areas (40x)<br />
Suboptimal (S) at least 5 partially preserved marrow particles<br />
Inadequate (I) fewer than 5 partially preserved marrow particles<br />
Aspirate:<br />
Adequate (A) 3 or more spicules, 300 or more cells<br />
Suboptimal (S) 1 or 2 spicules, 100 or more cells<br />
Inadequate (I) no spicules, BM diff. cannot be performed (less than 100 cells)<br />
Touch imprint:<br />
Adequate (A) 300 or more cells<br />
Suboptimal (S) 100 or more cells, less than 300<br />
Inadequate (I) BM diff. cannot be performed (less than 100 cells)<br />
V. Dictations, pro<strong>of</strong> reading, and final sign-out<br />
a. After the initial review <strong>of</strong> a case with the hematopathologist, please dictate<br />
the report to the best <strong>of</strong> your ability. Additional studies and final diagnosis<br />
can be added later.<br />
b. After you have dictated the final report, pro<strong>of</strong> read it, and then arrange with the<br />
faculty how they wish you to proceed. Some would like the slides and<br />
paperwork.<br />
c. Faculty specific requests.<br />
<br />
Dr. Roth: For normal flow interpretations, please use “CGR_NEG_BM”<br />
and modify appropriately<br />
VI.<br />
Wednesday 8:30 am conference<br />
a. The trainee on the adult bone marrow service is responsible for the 3 cases<br />
for the Wednesday conference for the following week after sign-out with the<br />
faculty member on BM#1 service.<br />
b. If there is a trainee on the pediatric/ATL bone marrow service, then the trainees<br />
should determine if that trainee is presenting a pediatric or ATL case.<br />
c. If the trainee(s) cannot meet this expectation, it is his, her or their<br />
responsibility to make arrangements with someone, such as the faculty<br />
member, so that 3 cases get presented.<br />
d. Discuss the last minute details <strong>of</strong> your cases to be presented with the<br />
responsible faculty member on Monday, two days before the conference.<br />
Determine if the faculty member needs to review the digital images or any other<br />
details.
VII.<br />
Going <strong>of</strong>f service<br />
a. Please make it clear to the responsible faculty member what the status <strong>of</strong> a case<br />
is, what is pending, and where you have put it.<br />
b. At the beginning <strong>of</strong> this last week on service, discuss with the responsible faculty<br />
member who will be presenting at Wednesday 8:30 am conference. In most<br />
cases, you will still be able to present your cases even if on another<br />
hematopathology service.<br />
Policy for Review <strong>of</strong> Bone Marrow Aspirates, Particle Preparations and Biopsies<br />
Technologists will screen all smears; assess quality <strong>of</strong> specimen and quality <strong>of</strong> stain.<br />
Technologist will inform Trainee or if no trainee, faculty when new marrow aspirates with<br />
acute leukemia or day 14 S/P chemotherapy are ready to be reviewed. A worksheet<br />
with the CBC data and morphology comments written in should be printed and made<br />
available for the review with the aspirate smear and PB smear. In addition a working<br />
draft that includes previous cases will be attached. In other cases check review bin in<br />
the sign-out room.<br />
Trainee/Pathologist will review aspirate smears/cases together with the history and other<br />
clinical data on day they are received in the laboratory.<br />
Clinicians performing marrows may also request a marrow differential or iron stain. This<br />
will have been noted by the technologist on the form that is used to record what special<br />
studies are being requested.<br />
Other useful information<br />
1. To order additional immunohistochemical stains, FISH molecular orders and all other non<br />
stat requests on bone marrow cases call the Audix stain line 412-802-3273 and leave a<br />
message. These are routinely picked up during normal working hours.<br />
2. Prior slides:<br />
‣ Bone marrow slides from part <strong>of</strong> 2009-present are in G325.1.<br />
‣ Bone marrow slides from unknown-part <strong>of</strong> 2008 are at Iron Mountain.(see table<br />
below)<br />
‣ In the Hematology Lab, the peripheral blood slides are kept for 2 weeks and are filed<br />
by accession number. The CBC results are in Sunquest/Mysis.<br />
‣ Slides to preview for bone marrow service #1 are placed in G315. There is<br />
also a bin for the cases ready for the technologist. Cases ready for sign out<br />
are also placed in G315.<br />
Flow reports are tubed to the bone marrow room G325.1 up to 5:15pm. After that, urgent cases<br />
are delivered directly to the pathologist
APPENDIX C LOCATION OF BONE MARROW RECORDS<br />
CHP UPMC MUH (PRIOR TO MERGER)<br />
SLIDES REPORTS<br />
SLIDES<br />
REPORTS<br />
SLIDES REPORTS<br />
1994 – 2008<br />
(PHB08-2859)<br />
In Iron mountain<br />
PHB08-2860 to<br />
present : see<br />
adult slides<br />
locations.<br />
1996-Present:<br />
In CoPath<br />
Client Server.<br />
Part 2009-Present:<br />
PHB<br />
(09-4561- present)<br />
G325.1 PUH<br />
Bone Marrow Room.<br />
PHB<br />
08-1742 to 09-4560<br />
REPORTS<br />
1990-Present<br />
In Co-path<br />
Client Server.<br />
Starting April, 2009<br />
Surg path reqs<br />
stored in the Scaife<br />
processing area.<br />
1983-1988 asp<br />
1991-1995 asp<br />
and bx:<br />
Iron Mountain.<br />
Unknown-1991 bx:<br />
MUH Surgical<br />
Pathology files<br />
in Iron Mountain.<br />
1990-1995:<br />
As described<br />
for UPMC<br />
In S764 Scaife Hall<br />
(flow processing area)<br />
Unknown-part 2008<br />
(PHB08-1741)<br />
Iron Mountain.<br />
1981-1990:<br />
A Stem 6th floor,<br />
Micr<strong>of</strong>iche files<br />
1967-1982 asp:<br />
Iron Mountain.<br />
Unknown-<br />
1990:<br />
BRM<br />
1976-1994:<br />
Iron Mountain.<br />
Unknown-1976<br />
CHP Pathology<br />
(basement)<br />
1930-1938,<br />
1950-1996:<br />
BRM<br />
1963-1981:<br />
BRM
Forms and Templates for<br />
Bone Marrow Service
ADULT BONE MARROW SERVICE REQUEST FORM<br />
PHB13- Date ADDSYNOPTIC<br />
Name:<br />
Requests:<br />
PB not available<br />
Do full PB Diff and review<br />
Scan PB for morphology, blasts and abnormal cells<br />
Scan PB for blasts and abnormal cells<br />
Scan BM smear for tumor<br />
Do BM Diff with detailed review <strong>of</strong> smears<br />
300 cell count 500 cell count<br />
*default is 300 cell differential<br />
Order Iron Stain on biopsy/smear<br />
Order the following cytochemical stains:<br />
SB PX PAS CAE DBLE (+/-fl)<br />
NSE (Acetate Esterase +/- fluoride)<br />
NSE (Butyrate Esterase +/- fluoride)<br />
Order the following other stains:<br />
Stain Specimen Date/Time Tech<br />
(Bx,FBM,clot...)<br />
_______________________ ________ ___________________<br />
_______________________ ________ ___________________<br />
Molecular:<br />
Other:__________________________________________________<br />
Print full report<br />
Pull slides<br />
Most recent previous BM <br />
First diagnostic BM <br />
PH__-___________ <br />
PH__-___________ <br />
_____________________________<br />
Pathologist/<strong>Resident</strong><br />
#1 or #2 or #3 or CHP Bone Marrow Service<br />
---------------------------------------------------------------------------------------------------------------------<br />
Quality Assurance Check:<br />
Satisfactory.<br />
Less than optimal:<br />
stain quality:_______________________________<br />
differential count PB _ BM _ : __________<br />
<br />
<br />
<br />
morphology evaluation :______________________<br />
history incomplete/incorrect :__________________<br />
Comment
ADULT BONE MARROW WORKSHEET<br />
OUTSIDE ADULT BONE MARROW WORKSHEET<br />
Technologist: ________________________________________________________________________<br />
Clinical History:<br />
______________________________________________________________________________________<br />
______________________________________________________________________________________<br />
______________________________________________________________________________________<br />
______________________________________________________________________________________<br />
Medications/Chemotherapy/Growth Factors:______________________________________________<br />
______________________________________________________________________________________<br />
DIAGNOSIS:<br />
Part 1) PERIPHERAL BLOOD -_________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
Parts 2&3) BONE MARROW, BIOPSY,(TOUCH IMPRINTS) AND ASPIRATE (AND PARTICLE<br />
PREPARATION)-<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_________________________________________________________________ (See Comment)<br />
COMMENT:_____________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
DESCRIPTION:<br />
PERIPHERAL BLOOD:<br />
The following CBC is from ___/___/___, SLIDE # ___________<br />
CBC Patient Typical Normal Reference Range<br />
. Value (Male/Female)<br />
WBC ____________x10E+9/L (4.40 - 11.3)<br />
RBC ____________x10E+12/L (4.50 - 5.90/4.10 - 5.10)<br />
Hgb ____________g/dl (14.0 - 17.5/12.3 - 15.3)<br />
Hct ____________% (41.5 - 50.4/35.9 - 44.6)<br />
MCV ____________fl (80.0 - 96.0)<br />
MCH ____________pg (27.5 - 33.2)<br />
MCHC ____________gm/dl (33.4 - 35.5)<br />
RDW ____________%<br />
PLT ____________x10E+9/L ( 150 - 450 )<br />
Retic ___________% ( 0.5 - 1.5 )<br />
Retic ___________x10E+12/L (0.025 - 0.075)<br />
Polys ___________% __________(ABS) (1.80 - 7.80)<br />
Bands ___________% __________(ABS) (0.00 - 0.70)<br />
Lymphs ___________% __________(ABS) (1.00 - 4.80)<br />
Atyp. lymph _________% __________(ABS)<br />
Monos ___________% __________(ABS) (0.00 - 0.80)<br />
Eos ___________% __________(ABS) (0.00 - 0.45)<br />
Baso ___________% __________(ABS) (0.00 - 0.20)<br />
Blasts ___________% __________(ABS)
Promyel ___________% __________(ABS)<br />
Myelo ___________% __________(ABS)<br />
Meta ___________% __________(ABS)<br />
NRBC/100 WBC ___________<br />
The peripheral blood smear was reviewed as follows:<br />
______________________________________________________________________________<br />
______________________________________________________________________________<br />
RED BLOOD CELL MORPHOLOGY:<br />
___Acanthocytes, ___Anisocytosis, ___Basophilic Stippling, ___Burr Cells,<br />
___Howell-Jolly Bodies, ___Hypochromia, ___Macrocytes, ___Microcytes,<br />
___Normochromic, ___Normocytic, ___Ovalocytes, ___Poikilocytosis,<br />
___Polychromasia, ___Schistocytes, ___Spherocytes, ___Target Cells, ___Teardrops,<br />
___Unremarkable,<br />
_______________________________________________________________<br />
________________________________________________________________________________<br />
WHITE BLOOD CELL MORPHOLOGY:<br />
_____Auer Rods, _____Blasts, _____Dohle Bodies, _____Hypogranularity,<br />
_____Hypersegmentation, _____Pseudo-Pelger-Huet Anomaly, _____Toxic Granulation,<br />
_____Atypical Lymphocytes, _____Unremarkable, _____Vacuoles _____________________<br />
_________________________________________________________________________________<br />
PLATELETS: are (increased, decreased, adequate) with (clumping, giant forms) seen.<br />
_________________________________________________________________________________<br />
BONE MARROW:<br />
ADEQUACY STATEMENT:<br />
The MARROW ASPIRATE SMEARS are (ADEQUATE, SUBOPTIMAL, INADEQUATE) for<br />
interpretation precluding a differential). ( ___ aspirate smears and _____ touch<br />
imprints reviewed).<br />
The MARROW BIOPSY is (ADEQUATE, SUBOPTIMAL, INADEQUATE) for interpretation.<br />
THE MARROW PARTICLE PREP is (ADEQUATE, INADEQUATE) for interpretation.<br />
“Evaluation <strong>of</strong> the bone marrow biopsy includes review <strong>of</strong> an H&E stain as<br />
well as a PAS stain which is done, in part, to highlight the myeloids and<br />
megakaryocytic elements, further evaluate the myeloid:erythroid ratio and<br />
evaluate the underlying bone marrow stroma.”<br />
BONE MARROW differential (_____ cells counted):<br />
Bone Marrow Patient Adult Normal<br />
Differential Value Mean Range<br />
Blast _____________% 1.0 ( 0.0 - 2.0 )<br />
Promyelocyte _____________% 3.0 ( 2.0 - 4.0 )<br />
Myelocyte _____________% 12.0 ( 8.0 - 16.0 )<br />
Metamyelocyte _____________% 17.0 ( 10.0 - 25.0 )<br />
Band _____________% 12.0 ( 9.0 - 18.0 )<br />
PMN _____________% 9.0 ( 7.0 - 14.0 )<br />
Eos Myelo/Meta _____________% 2.0 ( 1.0 - 4.0 )<br />
Eos Band _____________% 1.0 ( 0.0 - 3.0 )<br />
Eos Seg _____________% 1.0 ( 1.0 - 2.0 )<br />
Basophil _____________% 0.0 ( 0.0 - 0.2 )<br />
Monocytes _____________% 1.0 ( 0.0 - 2.0 )<br />
Pronormoblasts _____________% 1.0 ( 0.0 - 1.0 )<br />
Normoblasts _____________% 24.0 ( 16.0 - 32.0 )
Lymphocytes _____________% 16.0 ( 11.0 - 23.0 )<br />
Plasma Cells _____________% 2.0 ( 0.0 - 3.0 )<br />
Other<br />
_____________%<br />
Myeloid/Erythroid _____________% 2.4 ( 1.5 - 3.3 )<br />
The MARROW is (mildly, moderately, markedly) (normocellular, hypocellular,<br />
hypercellular)<br />
(_____% cellular). There is stromal injury/chemotherapy effect.<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
The MYELOID/ERYTHROID RATIO is (mildly, moderately, markedly)(increased, decreased,<br />
unremarkable, not evaluable)<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
ERYTHROID MATURATION is (complete, slight, moderate, marked, megaloblastoid,<br />
megaloblastic, asynchronous, with dyserythropoietic forms).<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
MYELOID MATURATION is (complete, slight, moderate, marked, megaloblastoid,<br />
megaloblastic, with dysplastic forms, with pseudo-Pelger-Huet forms, with a left<br />
shift).<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
MEGAKARYOCYTES are (present in, absent) (mildly, moderately, markedly) (increased,<br />
decreased, normal) numbers (with clusters, aggregates, very large aggregates present).<br />
MEGAKARYOCYTES<br />
include (monolobate, hypersegmented, small, non-budding, dysplastic) forms.<br />
____________________________________________________________________________________<br />
____________________________________________________________________________________<br />
STAINABLE IRON is (absent, decreased, present, moderately abundant, abundant) based on<br />
an iron stain performed on the (aspirate smear, biopsy, particle preparation).<br />
There are (no, rare, moderate numbers <strong>of</strong>, numerous) ringed sideroblasts identified.<br />
No focal lesions are identified. The bony trabeculae are (unremarkable, thinned,<br />
show osteoclastic resorption, osteoblastic rimming, show new bone formation).<br />
____________________________________________________________________________________<br />
____________________________________________________________________________________<br />
ANCILLARY STUDIES:<br />
FLOW/IHC1: Medical necessity justification for the immunohistochemical stains that<br />
were needed in addition to the flow cytometric immunophenotypic studies for the best<br />
diagnosis possible is as follows:<br />
The flow cytometric studies did not define the precise nature <strong>of</strong> all the cellular<br />
elements <strong>of</strong> concern in this specimen.<br />
FLOW/IHC2: Medical necessity justification for the immunohistochemical stains that<br />
were needed in addition to the flow cytometric immunophenotypic studies for the best<br />
diagnosis possible is as follows:<br />
The flow cytometric studies are not clearly representative <strong>of</strong> all the features<br />
requiring evaluation in this specimen.
ADULT BONE MARROW WORKSHEET<br />
INSIDE ADULT BONE MARROW WORKSHEET<br />
Technologist: ________________________________________________________________________<br />
Clinical History:<br />
______________________________________________________________________________________<br />
______________________________________________________________________________________<br />
______________________________________________________________________________________<br />
______________________________________________________________________________________<br />
Medications/Chemotherapy/Growth Factors:______________________________________________<br />
______________________________________________________________________________________<br />
DIAGNOSIS:<br />
Part 1) PERIPHERAL BLOOD -_________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
Parts 2&3) BONE MARROW, BIOPSY,(TOUCH IMPRINTS) AND ASPIRATE (AND PARTICLE<br />
PREPARATION)-<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_________________________________________________________________ (See Comment)<br />
COMMENT:_____________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
DESCRIPTION:<br />
PERIPHERAL BLOOD:<br />
The following CBC is from ___/___/___, SLIDE # ___________<br />
CBC Patient UPMC-Presbyterian Normal Range<br />
. Value (Male/Female)<br />
WBC ____________x10E+9/L (3.8 - 10.6)<br />
RBC ____________x10E+12/L (4.13 - 5.57/3.73 - 4.89)<br />
Hgb ____________g/dl (12.9 - 16.9/11.6 - 14.6)<br />
Hct ____________% (38.0 - 48.8/34.1 - 43.3)<br />
MCV ____________fl (82.6 - 97.4)<br />
MCH ____________pg (27.8 - 33.4)<br />
MCHC ____________gm/dl (32.7 - 35.5)<br />
RDW ____________% (11.8 - 15.2)<br />
PLT ____________x10E+9/L ( 156 - 369 )<br />
Retic ___________% ( 0.8 - 2.0/0.8 - 4.0)<br />
Retic ___________x10E+12/L (0.018 - 0.158)<br />
Polys ___________% __________(ABS) (2.24 - 7.68)<br />
Bands ___________% __________(ABS) (0.10 - 0.80)<br />
Lymphs ___________% __________(ABS) (0.80 - 3.65)<br />
Atyp. lymph _________% __________(ABS)<br />
Monos ___________% __________(ABS) (0.30 - 0.90)<br />
Eos ___________% __________(ABS) (0.00 - 0.40)<br />
Baso ___________% __________(ABS) (0.00 - 0.06)
Blasts ___________% __________(ABS)<br />
Promyel ___________% __________(ABS)<br />
Myelo ___________% __________(ABS)<br />
Meta ___________% __________(ABS)<br />
NRBC/100 WBC ___________<br />
The peripheral blood smear was reviewed as follows:<br />
______________________________________________________________________________<br />
______________________________________________________________________________<br />
RED BLOOD CELL MORPHOLOGY:<br />
___Acanthocytes, ___Anisocytosis, ___Basophilic Stippling, ___Burr Cells,<br />
___Howell-Jolly Bodies, ___Hypochromia, ___Macrocytes, ___Microcytes,<br />
___Normochromic, ___Normocytic, ___Ovalocytes, ___Poikilocytosis,<br />
___Polychromasia, ___Schistocytes, ___Spherocytes, ___Target Cells, ___Teardrops,<br />
___Unremarkable, _______________________________________________________________<br />
________________________________________________________________________________<br />
WHITE BLOOD CELL MORPHOLOGY:<br />
_____Auer Rods, _____Blasts, _____Dohle Bodies, _____Hypogranularity,<br />
_____Hypersegmentation, _____Pseudo-Pelger-Huet Anomaly, _____Toxic Granulation,<br />
_____Atypical Lymphocytes, _____Unremarkable, _____Vacuoles _____________________<br />
_________________________________________________________________________________<br />
PLATELETS: are (increased, decreased, adequate) with (clumping, giant forms) seen.<br />
_________________________________________________________________________________<br />
BONE MARROW:<br />
ADEQUACY STATEMENT:<br />
The MARROW ASPIRATE SMEARS are (ADEQUATE, SUBOPTIMAL, INADEQUATE) for<br />
interpretation precluding a differential). (___ aspirate smears and _____ touch<br />
imprints reviewed).<br />
The MARROW BIOPSY is (ADEQUATE, SUBOPTIMAL, INADEQUATE) for interpretation.<br />
THE MARROW PARTICLE PREP is (ADEQUATE, INADEQUATE) for interpretation.<br />
“Evaluation <strong>of</strong> the bone marrow biopsy includes review <strong>of</strong> an H&E stain as<br />
well as a PAS stain which is done, in part, to highlight the myeloids and<br />
megakaryocytic elements, further evaluate the myeloid:erythroid ratio and<br />
evaluate the underlying bone marrow stroma.”<br />
BONE MARROW differential (_____ cells counted):<br />
Bone Marrow Patient Adult Normal<br />
Differential Value Mean Range<br />
Blast _____________% 1.0 ( 0.0 - 2.0 )<br />
Promyelocyte _____________% 3.0 ( 2.0 - 4.0 )<br />
Myelocyte _____________% 12.0 ( 8.0 - 16.0 )<br />
Metamyelocyte _____________% 17.0 ( 10.0 - 25.0 )<br />
Band _____________% 12.0 ( 9.0 - 18.0 )<br />
PMN _____________% 9.0 ( 7.0 - 14.0 )<br />
Eos Myelo/Meta _____________% 2.0 ( 1.0 - 4.0 )<br />
Eos Band _____________% 1.0 ( 0.0 - 3.0 )<br />
Eos Seg _____________% 1.0 ( 1.0 - 2.0 )<br />
Basophil _____________% 0.0 ( 0.0 - 0.2 )<br />
Monocytes _____________% 1.0 ( 0.0 - 2.0 )<br />
Pronormoblasts _____________% 1.0 ( 0.0 - 1.0 )<br />
Normoblasts _____________% 24.0 ( 16.0 - 32.0 )<br />
Lymphocytes _____________% 16.0 ( 11.0 - 23.0 )
Plasma Cells _____________% 2.0 ( 0.0 - 3.0 )<br />
Other<br />
_____________%<br />
Myeloid/Erythroid _____________% 2.4 ( 1.5 - 3.3 )<br />
The MARROW is (mildly, moderately, markedly) (normocellular, hypocellular,<br />
hypercellular)<br />
(_____% cellular). There is stromal injury/chemotherapy effect.<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
The MYELOID/ERYTHROID RATIO is (mildly, moderately, markedly)(increased, decreased,<br />
unremarkable, not evaluable)<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
ERYTHROID MATURATION is (complete, slight, moderate, marked, megaloblastoid,<br />
megaloblastic, asynchronous, with dyserythropoietic forms).<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
MYELOID MATURATION is (complete, slight, moderate, marked, megaloblastoid,<br />
megaloblastic, with dysplastic forms, with pseudo-Pelger-Huet forms, with a left<br />
shift).<br />
_____________________________________________________________________________________<br />
_____________________________________________________________________________________<br />
MEGAKARYOCYTES are (present in, absent) (mildly, moderately, markedly) (increased,<br />
decreased, normal) numbers (with clusters, aggregates, very large aggregates present).<br />
MEGAKARYOCYTES<br />
include (monolobate, hypersegmented, small, non-budding, dysplastic) forms.<br />
____________________________________________________________________________________<br />
____________________________________________________________________________________<br />
STAINABLE IRON is (absent, decreased, present, moderately abundant, abundant) based on<br />
an iron stain performed on the (aspirate smear, biopsy, particle preparation).<br />
There are (no, rare, moderate numbers <strong>of</strong>, numerous) ringed sideroblasts identified.<br />
No focal lesions are identified. The bony trabeculae are (unremarkable, thinned,<br />
show osteoclastic resorption, osteoblastic rimming, show new bone formation).<br />
____________________________________________________________________________________<br />
____________________________________________________________________________________<br />
ANCILLARY STUDIES:<br />
FLOW/IHC1: Medical necessity justification for the immunohistochemical stains that<br />
were needed in addition to the flow cytometric immunophenotypic studies for the best<br />
diagnosis possible is as follows:<br />
The flow cytometric studies did not define the precise nature <strong>of</strong> all the cellular<br />
elements <strong>of</strong> concern in this specimen.<br />
FLOW/IHC2: Medical necessity justification for the immunohistochemical stains that<br />
were needed in addition to the flow cytometric immunophenotypic studies for the best<br />
diagnosis possible is as follows:<br />
The flow cytometric studies are not clearly representative <strong>of</strong> all the features<br />
requiring evaluation in this specimen.<br />
ANCILLARY STUDIES:<br />
Cytochemical Stains<br />
Histologic Section Immunohistochemistry/In situ Hybridization
CYTOCHEMICAL STAINS<br />
Interpretation/Diagnosis (for special tests only/addenda):______<br />
________________________________________________________________<br />
________________________________________________________________<br />
Results/Ancillary Studies:<br />
Cytochemical stains were performed on: _________________________<br />
The following results were found:<br />
Stain Usual Reactivity Results<br />
---------------------------------------------------------------------------------------------------------------<br />
Sudan Black gran, mono<br />
---------------------------------------------------------------------------------------------------------------<br />
Peroxidase<br />
gran, mono<br />
----------------------------------------------------------------------------------------------------------------<br />
PAS<br />
diff*-gran, mono<br />
block-lymph<br />
diff +/- block-abnl RBC<br />
-----------------------------------------------------------------------------------------------------------------<br />
CAE<br />
CAE-gran, mast<br />
-----------------------------------------------------------------------------------------------------------------<br />
NSE<br />
NSE-mono, mega<br />
NSE Positivity<br />
Was / was not<br />
inhibited by fluoride<br />
--------------------------------------------------------------------------------------------------------------------
DAY 14 BONE MARROW WORKSHEET<br />
Technologist:<br />
_________________________________________________________________<br />
Clinical History:<br />
__________________________________________________________________________________<br />
__________________________________________________________________________________<br />
__________________________________________________________________________________<br />
__________________________________________________________________________________<br />
Medications/Chemotherapy/Growth Factors:__________________________________________<br />
__________________________________________________________________________________<br />
Specimen adequacy: Aspirate: Adequate/Suboptimal/Inadequate/Not evaluable<br />
Touch imprint: Adequate/Suboptimal/Inadequate/Not evaluable<br />
Biopsy:<br />
Adequate/Suboptimal/Inadequate/Not evaluable<br />
Particle/clot: Adequate/Suboptimal/Inadequate/Not evaluable<br />
Bone marrow blasts: Absent/Present ( %)/Not evaluable<br />
Counted on aspirate/touch imprint:<br />
Cells counted (100, 200, 300, 500, other)<br />
Marrow cellularity: ( %)<br />
Peripheral blood blasts: Absent/Present( %)/Slide not available.<br />
Cells counted(100,200)<br />
Marrow regeneration:<br />
Erythropiesis: Absent/Rare/Present/Not evaluable<br />
Granulopoiesis: Absent/Rare/Present/Not evaluable<br />
Megakaryopoiesis: Absent/Rare/Present/Not evaluable<br />
Additional morphologic findings:<br />
Clusters <strong>of</strong> immature cells<br />
Lymphoid aggregate(s)<br />
Granuloma(s)<br />
Other:<br />
Immunohistochemical stains performed to better assess marrow findings:<br />
CD34<br />
CD117<br />
TDT<br />
Other<br />
“Medical necessity justification for the immunohistochemical stains that were needed in<br />
addition to the flow cytometric immunophenotypic studies for the best diagnosis possible is
as follows: The flow cytometric studies did not define the precise nature <strong>of</strong> all the<br />
cellular elements <strong>of</strong> concern in this specimen”.<br />
Cytochemical stains performed to better assess marrow findings:<br />
Myeloperoxidase<br />
Non-specific (Butyrate) esterase<br />
This report is based on morphologic evaluation <strong>of</strong> submitted bone marrow specimen and the<br />
following completed ancillary studies:<br />
attached)<br />
Immunohistochemical stains<br />
Cytochemical stains<br />
Flow cytometric analysis (separate report attached)<br />
Classical cytogenetic (karyotypic) analysis (separate report<br />
Cytogenetic FISH analysis (separate report attached)<br />
Molecular analysis (separate report attached)<br />
Other:<br />
The following studies are pending with results to follow:<br />
Immunohistochemical stains<br />
Cytochemical stains<br />
Flow cytometric analysis<br />
Classical cytogenetic (karyotypic) analysis<br />
Cytogenetic FISH analysis<br />
Molecular analysis<br />
Other:<br />
2 bone marrow aspirate smears and 1 touch prep reviewed.<br />
“Evaluation <strong>of</strong> the bone marrow biopsy includes review <strong>of</strong> an H&E stain as well as a PAS<br />
stain which is done, in part, to highlight the myeloids and megakaryocytic elements, further<br />
evaluate the myeloid: erythroid ratio and evaluate the underlying bone marrow stroma”.<br />
The following CBC is from ___/___/___, SLIDE # ___________<br />
CBC Patient UPMC-Presbyterian Normal Range<br />
. Value (Male/Female)<br />
WBC ___________ x10E+9/L (3.8 - 10.6)<br />
RBC ___________ x10E+12/L (4.13 - 5.57/3.73 - 4.89)<br />
Hgb ____________g/dl (12.9 - 16.9/11.6 - 14.6)<br />
Hct ____________% (38.0 - 48.8/34.1 - 43.3)<br />
MCV ___________ fl (82.6 - 97.4)<br />
MCH __________ pg (27.8 - 33.4)<br />
MCHC __________ gm/dl (32.7 - 35.5)<br />
RDW __________ % (11.8 - 15.2)<br />
PLT ___________ x10E+9/L ( 156 - 369 )<br />
Retic ___________% ( 0.8 - 2.0/0.8 - 4.0)<br />
Retic ___________x10E+12/L (0.018 - 0.158)<br />
Polys __________% __________(ABS) (2.24 - 7.68)<br />
Bands ___________% __________(ABS) (0.10 - 0.80)<br />
Lymphs ___________% __________(ABS) (0.80 - 3.65)<br />
Atyp. lymph _________% __________(ABS)<br />
Monos ___________% __________(ABS) (0.30 - 0.90)<br />
Eos __________% __________(ABS) (0.00 - 0.40)<br />
Baso __________% __________(ABS) (0.00 - 0.06)
Blasts _________% __________(ABS)<br />
Promyel __________% __________(ABS)<br />
Myelo __________% __________(ABS)<br />
Meta __________% __________(ABS)<br />
NRBC/100 WBC __________<br />
Recommendations for Consistent Terminology in <strong>Hematopathology</strong> Reports<br />
1. Use the 2008 WHO Classification (see the monograph)<br />
2. Leukemic follow-up marrows<br />
a. History should always state: Day status post chemotherapy (or status post<br />
transplant) for abbreviated disease name e.g. AML, promyelocytic<br />
i. If time unknown, delete “day .”<br />
*Remember day status post chemotherapy refers to days following initiation <strong>of</strong> therapy.<br />
A. Preferably a similar statement should also be in the diagnosis following the initial diagnostic line<br />
e.g.<br />
Bone marrow, biopsy and aspirate--<br />
A. Markedly hypocellular marrow with chemotherapy effect<br />
B. (Day 7 status post chemotherapy for AML).<br />
B. If history <strong>of</strong> leukemia is remote, history (and if desired, diagnosis) should state:<br />
“Status post previous diagnosis <strong>of</strong> _____________________”.<br />
3. Whenever appropriate, state, in comment, how the marrow compares to the immediate previous<br />
one and give the previous marrow number; e.g., “compared to the patient’s previous marrow<br />
(PHB11-XXXX), blasts are not increased.”<br />
4. Whenever any ancillary studies or special stains are reported, be sure to include an interpretation<br />
and not just a statement <strong>of</strong> the result. On cases without histologic material, be sure to dictate an<br />
interpretation including a summary <strong>of</strong> the results, which will be typed in the space where diagnosis<br />
usually goes.<br />
For example, if an addendum is issued with the results <strong>of</strong> a reticulin stain, do not include just,<br />
“The reticulin stain shows a marked increase in reticulin fibers”, also an interpretation<br />
(i.e. “This strongly supports the diagnosis in this case <strong>of</strong> a myeloproliferative<br />
neoplasm”).<br />
5. Abnormal plasma cell proliferations should be given as specific a diagnosis as possible.
A. Plasma cell myeloma (use if possible).<br />
B. Plasma cell neoplasm (if definite neoplasm, but uncertain if myeloma).<br />
C. Plasmacytosis (with qualifier if the diagnosis <strong>of</strong> a neoplasm cannot be made).<br />
D. Plasmacytoma (a non-myeloma plasma cell neoplasm).<br />
E. Remember, plasma cell dyscrasia includes non-neoplastic disorders as well as plasma cell<br />
neoplasms.
Case number:___________________________<br />
<strong>Resident</strong> Bone Marrow Differential count Worksheet<br />
(_______ cells counted)<br />
Blasts<br />
Promyelocytes<br />
Myelocytes<br />
Metamyelocytes<br />
Bands/Seg Neutrophils<br />
Erythroid cells<br />
Lymphocytes<br />
Eosinophils<br />
Basophils<br />
Monocytes<br />
Plasma cells<br />
Myeloid:erythroid ratio<br />
Signature____________________________________________________<br />
Date: ___________________________<br />
Pathologist initials ____________
Protocol for the Examination <strong>of</strong> Specimens From Patients With<br />
Hematopoietic Neoplasms Involving the Bone Marrow<br />
Based on AJCC/UICC TNM, 7 th Edition<br />
Protocol web posting date: June 2012<br />
Procedures<br />
Bone marrow aspiration<br />
Bone marrow core (trephine) biopsy<br />
Authors<br />
Jerry W. Hussong, MD, DDS, FCAP*<br />
Cedars-Sinai Medical Center, Los Angeles, California<br />
Daniel A. Arber, MD<br />
Stanford University School <strong>of</strong> Medicine, Stanford, California<br />
Kyle T. Bradley MD, MS, FCAP<br />
Emory University Hospital, Atlanta, Georgia<br />
Michael S. Brown, MD, FCAP<br />
Yellowstone Pathology Institute Inc, Billings, Montana<br />
Chung-Che Chang, MD, PhD, FCAP<br />
The Methodist Hospital, Houston, Texas<br />
Monica E. de Baca, MD, FCAP<br />
Physicians Laboratory Ltd, Sioux Falls, South Dakota<br />
David W. Ellis, MBBS, FRCPA<br />
Flinders Medical Centre, Bedford Park, South Australia<br />
Kathryn Foucar, MD, FCAP<br />
University <strong>of</strong> New Mexico, Albuquerque, New Mexico<br />
Eric D. Hsi, MD, FCAP<br />
Cleveland Clinic Foundation, Cleveland, Ohio<br />
Elaine S. Jaffe, MD<br />
National Cancer Institute, Bethesda, Maryland<br />
Michael Lill, MB, BS, FRACP, FRCPA<br />
Cedars-Sinai Medical Center, Los Angeles, California<br />
Stephen P. McClure, MD<br />
Presbyterian Pathology Group, Charlotte, North Carolina<br />
L. Jeffrey Medeiros, MD, FCAP<br />
MD Anderson Cancer Center, Houston, Texas<br />
Sherrie L. Perkins, MD, PhD, FCAP<br />
University <strong>of</strong> Utah Health Sciences Center, Salt Lake City, Utah<br />
For the Members <strong>of</strong> the Cancer Committee, College <strong>of</strong> American Pathologists<br />
* Denotes the primary and senior author. All other contributing authors are listed alphabetically.
Surgical Pathology Cancer Case Summary<br />
Protocol web posting date: June 2012<br />
BONE MARROW: Aspiration, Core (Trephine) Biopsy<br />
Select a single response unless otherwise indicated.<br />
Specimen (select all that apply) (Note A)<br />
___ Peripheral blood smear<br />
___ Bone marrow aspiration<br />
___ Bone marrow aspirate clot (cell block)<br />
___ Bone marrow core (trephine) biopsy<br />
___ Bone marrow core touch preparation (imprint)<br />
___ Other (specify): ___________________________<br />
___ Not specified<br />
Procedure (select all that apply)<br />
___ Aspiration<br />
___ Biopsy<br />
___ Other (specify): ___________________________<br />
___ Not specified<br />
Aspiration Site (if performed) (select all that apply) (Note B)<br />
___ Right posterior iliac crest<br />
___ Left posterior iliac crest<br />
___ Sternum<br />
___ Other (specify): ___________________________<br />
___ Not specified<br />
Biopsy Site (if performed) (select all that apply) (Note B)<br />
___ Right posterior iliac crest<br />
___ Left posterior iliac crest<br />
___ Other (specify): ___________________________<br />
___ Not specified<br />
Histologic Type (Note C)<br />
Note: The following is a partial list <strong>of</strong> the 2008 World Health Organization (WHO) classification 1 and includes those neoplasms<br />
seen in bone marrow specimens.<br />
___ Histologic type cannot be assessed<br />
Myeloproliferative Neoplasms<br />
___ Chronic myelogenous leukemia, BCR-ABL1 positive
___ Chronic neutrophilia leukemia<br />
___ Polycythemia vera<br />
___ Primary myel<strong>of</strong>ibrosis<br />
___ Essential thrombocythemia<br />
___ Chronic eosinophilic leukemia, not otherwise specified (NOS)<br />
___ Mastocytosis (specify type): ______________________<br />
___ Myeloproliferative neoplasm, unclassifiable<br />
Myeloid and Lymphoid Neoplasms With Eosinophilia and Abnormalities <strong>of</strong> PDGFRA, PDGFRB and FGFR1<br />
___ Myeloid or lymphoid neoplasm with PDGFRA rearrangement<br />
___ Myeloid neoplasm with PDGFRB rearrangement<br />
___ Myeloid or lymphoid neoplasm with FGFR1 abnormalities<br />
Myelodysplastic/Myeloproliferative Neoplasms<br />
___ Chronic myelomonocytic leukemia<br />
___ Atypical chronic myeloid leukemia BCR-ABL1 negative<br />
___ Juvenile myelomonocytic leukemia<br />
___ Myelodysplastic/myeloproliferative neoplasm, unclassifiable<br />
___ Refractory anemia with ring sideroblasts associated with marked thrombocytosis<br />
Myelodysplastic Syndromes<br />
___ Refractory anemia<br />
___ Refractory neutropenia<br />
___ Refractory thrombocytopenia<br />
___ Refractory anemia with ring sideroblasts<br />
___ Refractory cytopenia with multilineage dysplasia<br />
___ Refractory anemia with excess blasts<br />
___ Myelodysplastic syndrome associated with isolated del(5q)<br />
___ Myelodysplastic syndrome, unclassifiable<br />
___ Refractory cytopenia <strong>of</strong> childhood<br />
Acute Myeloid Leukemia (AML) With Recurrent Genetic Abnormalities<br />
___ AML with t(8;21)(q22;q22); RUNX1-RUNX1T1<br />
___ AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11<br />
___ Acute promyelocytic leukemia with t(15;17)(q22;q12); PML-RARA<br />
___ AML with t(9;11)(p22;q23); MLLT3-MLL<br />
___ AML with t(6;9)(p23;q34); DEK-NUP214<br />
___ AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2); RPN1-EVI1<br />
___ AML (megakaryoblastic) with t(1;22)(p13;q13); RBM15-MKL1<br />
___ AML with mutated NPM1<br />
___ AML with mutated CEBPA<br />
Acute Myeloid Leukemia With Myelodysplasia-Related Changes (select all that apply)<br />
___ Multilineage dysplasia<br />
___ Prior myelodysplastic syndrome<br />
___ Myelodysplasia-related cytogenetic abnormalities<br />
Therapy-Related Myeloid Neoplasms<br />
___ Therapy-related AML<br />
___ Therapy-related myelodysplastic syndrome<br />
___ Therapy-related myelodysplastic/myeloproliferative neoplasm<br />
Acute Myeloid Leukemia, NOS<br />
___ AML with minimal differentiation<br />
___ AML without maturation<br />
___ AML with maturation
___ Acute myelomonocytic leukemia<br />
___ Acute monoblastic/monocytic leukemia<br />
___ Acute erythroid leukemia<br />
___ Acute megakaryocytic leukemia<br />
___ Acute basophilic leukemia<br />
___ Acute panmyelosis with myel<strong>of</strong>ibrosis<br />
___ AML, NOS #<br />
Myeloid Proliferations Related to Down Syndrome<br />
___ Transient abnormal myelopoiesis<br />
___ Myeloid leukemia associated with Down syndrome<br />
Acute Leukemias <strong>of</strong> Ambiguous Lineage<br />
___ Acute undifferentiated leukemia<br />
___ Mixed phenotype acute leukemia with t(9;22)(q34;q11.2); BCR-ABL1<br />
___ Mixed phenotype acute leukemia with t(v;11q23); MLL rearranged<br />
___ Mixed phenotype acute leukemia, B/myeloid, NOS<br />
___ Mixed phenotype acute leukemia, T/myeloid, NOS<br />
___ Mixed phenotype acute leukemia, NOS, rare types (specify type): _____________<br />
___ Natural killer (NK) cell lymphoblastic leukemia/lymphoma<br />
Other Myeloid Leukemias<br />
___ Blastic plasmacytoid dendritic cell neoplasm<br />
Precursor Lymphoid Neoplasms<br />
___ B lymphoblastic leukemia/lymphoma, NOS #<br />
___ B lymphoblastic leukemia/lymphoma with t(9;22)(q34;q11.2); BCR-ABL1<br />
___ B lymphoblastic leukemia/lymphoma with t(v;11q23); MLL rearranged<br />
___ B lymphoblastic leukemia/lymphoma with t(12;21)(p13;q22); TEL-AML1 (ETV6-RUNX1)<br />
___ B lymphoblastic leukemia/lymphoma with hyperdiploidy<br />
___ B lymphoblastic leukemia/lymphoma with hypodiploidy (hypodiploid ALL)<br />
___ B lymphoblastic leukemia/lymphoma with t(5;14)(q31;q32); IL3-IGH<br />
___ B lymphoblastic leukemia/lymphoma with t(1;19)(q23;p13.3); E2A-PBX1 (TCF3-PBX1)<br />
___ T lymphoblastic leukemia/lymphoma<br />
Mature B-Cell Neoplasms<br />
___ Chronic lymphocytic leukemia/small lymphocytic lymphoma<br />
___ B-cell prolymphocytic leukemia<br />
___ Splenic B-cell marginal zone lymphoma<br />
___ Hairy cell leukemia<br />
___ Splenic B-cell lymphoma/leukemia, unclassifiable<br />
___ Splenic diffuse red pulp small B-cell lymphoma<br />
___ Hairy cell leukemia-variant<br />
___ Lymphoplasmacytic lymphoma<br />
___ Plasma cell myeloma<br />
___ Extranodal marginal zone lymphoma <strong>of</strong> mucosa-associated lymphoid tissue (MALT lymphoma)<br />
___ Follicular lymphoma<br />
___ Mantle cell lymphoma<br />
___ Diffuse large B-cell lymphoma (DLBCL), NOS<br />
___ T cell/histiocyte-rich large B-cell lymphoma<br />
___ Primary cutaneous DLBCL, leg type
___ Epstein-Barr virus (EBV)-positive DLBCL <strong>of</strong> the elderly<br />
___ DLBCL associated with chronic inflammation<br />
___ Lymphomatoid granulomatosis<br />
___ Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma<br />
___ Plasmablastic lymphoma<br />
___ Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease<br />
___ Burkitt lymphoma<br />
___ B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma<br />
and Burkitt lymphoma<br />
___ B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma<br />
and classical Hodgkin lymphoma<br />
___ B-cell lymphoma, NOS<br />
___ Other (specify): ____________________________<br />
Mature T- and NK-cell Neoplasms<br />
___ T-cell lymphoma, subtype cannot be determined (Note: not a category within the WHO<br />
classification)<br />
___ T-cell prolymphocytic leukemia<br />
___ T-cell large granular lymphocytic leukemia<br />
___ Chronic lymphoproliferative disorder <strong>of</strong> NK cells<br />
___ Aggressive NK-cell leukemia<br />
___ Adult T-cell leukemia/lymphoma<br />
___ Extranodal NK/T-cell lymphoma, nasal type<br />
___ Enteropathy-associated T-cell lymphoma<br />
___ Hepatosplenic T-cell lymphoma<br />
___ Mycosis fungoides<br />
___ Peripheral T-cell lymphoma, NOS<br />
___ Angioimmunoblastic T-cell lymphoma<br />
___ Anaplastic large cell lymphoma, ALK-positive<br />
___ Anaplastic large cell lymphoma, ALK-negative<br />
Hodgkin Lymphoma<br />
___ Nodular lymphocyte predominant Hodgkin lymphoma<br />
___ Classical Hodgkin lymphoma<br />
Histiocytic and Dendritic Cell Neoplasms<br />
___ Histiocytic sarcoma<br />
___ Langerhans cell histiocytosis<br />
___ Langerhans cell sarcoma<br />
___ Interdigitating dendritic cell sarcoma<br />
___ Follicular dendritic cell sarcoma<br />
___ Disseminated juvenile xanthogranuloma<br />
___ Histiocytic neoplasm, NOS<br />
Posttransplant Lymphoproliferative Disorders (PTLD) ##<br />
Early lesions:<br />
___ Plasmacytic hyperplasia<br />
___ Infectious mononucleosis-like PTLD<br />
___ Polymorphic PTLD<br />
___ Monomorphic PTLD (B- and T/NK-cell types)<br />
Specify subtype: ________________________
___ Classical Hodgkin lymphoma type PTLD ###<br />
___ Other (specify): ____________________________<br />
Note: Italicized histologic types denote provisional entities in the 2008 WHO classification.<br />
# An initial diagnosis <strong>of</strong> “AML, NOS” or “B lymphoblastic leukemia/lymphoma, NOS” may need to be given before the cytogenetic<br />
results are available or for cases that do not meet criteria for other leukemia subtypes.<br />
## These disorders are listed for completeness, but not all <strong>of</strong> them represent frank lymphomas.<br />
### Classical Hodgkin lymphoma type PTLD can be reported using either this protocol or the separate College <strong>of</strong> American<br />
Pathologists protocol for Hodgkin lymphoma. 2<br />
+ Additional Pathologic Findings<br />
+ Specify: _______________________________________<br />
+ Cytochemical/Special Stains (Note D)<br />
+ ___ Performed<br />
+ Specify stains and results: __________________________________<br />
______________________________________________________<br />
+ ___ Not performed<br />
Immunophenotyping (flow cytometry and/or immunohistochemistry) (Note E)<br />
___ Performed, see separate report: ___________________<br />
___ Performed<br />
Specify method(s) and results: ______________________________<br />
_____________________________________________________<br />
___ Not performed<br />
Cytogenetic Studies (Note F)<br />
___ Performed, see separate report: ___________________<br />
___ Performed<br />
Specify method(s) and results: ______________________________<br />
_____________________________________________________<br />
___ Not performed<br />
+ Fluorescence In Situ Hybridization (Note F)<br />
+ ___ Performed, see separate report: ___________________<br />
+ ___ Performed<br />
+ Specify method(s) and results: ______________________________<br />
_____________________________________________________<br />
+ ___ Not performed
+ Molecular Genetic Studies (Note F)<br />
+ ___ Performed, see separate report: ___________________<br />
+ ___ Performed<br />
Specify method(s) and results: ______________________________<br />
_____________________________________________________<br />
+ ___ Not performed<br />
+ Comment(s)<br />
+ Data elements preceded by this symbol are not required. However, these elements may be<br />
clinically important but are not yet validated or regularly used in patient management.
Explanatory Notes<br />
A. Specimen<br />
Complete evaluation <strong>of</strong> hematopoietic disorders involving the bone marrow requires integration<br />
<strong>of</strong> multiple pieces <strong>of</strong> data, including the clinical history, pertinent laboratory studies (eg,<br />
complete blood count [CBC], serum lactate dehydrogenase [LDH], and beta-2-microglobulin<br />
levels, serum protein electrophoresis, and immun<strong>of</strong>ixation results), and a satisfactory peripheral<br />
blood smear and bone marrow specimen. In most instances, this requires receiving a<br />
peripheral blood smear, bone marrow aspirate specimen, an aspirate clot section (cell block),<br />
and a bone marrow core biopsy. 3 Touch preparations (imprints) <strong>of</strong> the biopsy specimen are<br />
also very helpful. The World Health Organization (WHO) classification recommends performing<br />
a 200-cell differential count on peripheral blood smears and a 500-cell differential count on bone<br />
marrow aspirate specimens in the evaluation <strong>of</strong> hematopoietic disorders. 1 This will allow<br />
adequate evaluation <strong>of</strong> the cellular elements within the peripheral blood and bone marrow.<br />
In addition, submission <strong>of</strong> bone marrow (usually aspirate) material for flow cytometry<br />
immunophenotyping, cytogenetic studies, fluorescence in situ hybridization (FISH), and<br />
molecular studies is <strong>of</strong>ten necessary. The guidelines that follow are suggested for handling <strong>of</strong><br />
bone marrow specimens:<br />
The number <strong>of</strong> stained and unstained peripheral blood, bone marrow aspirate, and bone<br />
marrow core biopsy touch preparation smears should be recorded.<br />
<br />
<br />
<br />
<br />
<br />
The length <strong>of</strong> the bone marrow core biopsy(s) should be recorded.<br />
For conventional cytogenetic studies, a bone marrow aspirate specimen received in a<br />
sodium heparin tube is ideal, but fresh specimens submitted in saline or RPMI transport<br />
medium is sufficient.<br />
For immunophenotyping by flow cytometry, a bone marrow aspirate specimen received in an<br />
ACD tube (yellow top tube) or EDTA tube (lavender top tube) is preferred.<br />
Bone marrow core biopsy specimens require decalcification, and care must be taken not to<br />
under- or over-decalcify the specimen, as it will impact the ability to cut and interpret the<br />
histologic sections and may interfere with immunohistochemical staining. Formic acid<br />
decalcification procedures can also degrade DNA, whereas EDTA decalcification may allow<br />
for preservation <strong>of</strong> DNA for polymerase chain reaction (PCR) studies. EDTA decalcification,<br />
however, is slower than acid decalcification techniques.<br />
Fixation:<br />
o<br />
o<br />
o<br />
Zinc formalin or B5 fixatives produce superior cytologic detail but are not suitable for<br />
DNA extraction and impair some immunostains (eg, CD30). B5 has the additional<br />
limitation <strong>of</strong> requiring proper hazardous-materials disposal.<br />
Formalin fixation is preferable in many situations, as it allows for many ancillary tests<br />
such as molecular/genetic studies, in-situ hybridization, and immunophenotyping.<br />
Over-fixation (ie, more than 24 hours in formalin, more than 4 hours in zinc formalin or<br />
B5) should be avoided for optimal immunophenotypic reactivity.<br />
Care must be taken to ensure that high-quality specimens and sections are obtained for each<br />
bone marrow specimen. Often this requires working hand-in-hand with clinical colleagues to<br />
achieve this goal. In addition to being used for the diagnosis <strong>of</strong> primary hematopoietic<br />
disorders, bone marrow examination is <strong>of</strong>ten utilized as part <strong>of</strong> the pathologic staging <strong>of</strong> many<br />
hematopoietic neoplasms, including Hodgkin and non-Hodgkin lymphomas. 3-5 Bone marrow<br />
involvement identified within staging biopsy specimens typically indicates stage IV disease<br />
within the Ann Arbor staging system utilized by the American Joint Committee on Cancer<br />
(AJCC) 6 and the International Union Against Cancer (UICC). 7 For multiple myeloma, the Durie-
Salmon staging system is recommended by the AJCC. 8 Both staging systems are shown<br />
below. In pediatric patients, the St. Jude staging system is commonly used. 9<br />
AJCC/UICC Staging for Non-Hodgkin Lymphomas<br />
Stage I<br />
Stage II<br />
Stage III<br />
Stage IV<br />
Involvement <strong>of</strong> a single lymph node region (I), or localized involvement <strong>of</strong> a<br />
single extralymphatic organ or site in the absence <strong>of</strong> any lymph node<br />
involvement (IE). # , ##<br />
Involvement <strong>of</strong> 2 or more lymph node regions on the same side <strong>of</strong> the diaphragm<br />
(II), or localized involvement <strong>of</strong> a single extralymphatic organ or site in<br />
association with regional lymph node involvement with or without involvement <strong>of</strong><br />
other lymph node regions on the same side <strong>of</strong> the diaphragm (IIE). ## , ###<br />
Involvement <strong>of</strong> lymph node regions on both sides <strong>of</strong> the diaphragm (III), which<br />
also may be accompanied by extralymphatic extension in association with<br />
adjacent lymph node involvement (IIIE) or by involvement <strong>of</strong> the spleen (IIIS) or<br />
both (IIIE+S). ## , ### ,^<br />
Diffuse or disseminated involvement <strong>of</strong> 1 or more extralymphatic organs, with or<br />
without associated lymph node involvement; or isolated extralymphatic organ<br />
involvement in the absence <strong>of</strong> adjacent regional lymph node involvement, but in<br />
conjunction with disease in distant site(s). Stage IV includes any involvement <strong>of</strong><br />
the liver, bone marrow, or nodular involvement <strong>of</strong> the lung(s) or cerebral spinal<br />
fluid. ## , ### ,^<br />
# Multifocal involvement <strong>of</strong> a single extralymphatic organ is classified as stage IE and not stage<br />
IV.<br />
## For all stages, tumor bulk greater than 10 to 15 cm is an unfavorable prognostic factor.<br />
### The number <strong>of</strong> lymph node regions involved may be indicated by a subscript: eg, II 3 . For<br />
stages II to IV, involvement <strong>of</strong> more than 2 sites is an unfavorable prognostic factor.<br />
^ For stages III to IV, a large mediastinal mass is an unfavorable prognostic factor.<br />
Note: Direct spread <strong>of</strong> a lymphoma into adjacent tissues or organs does not influence<br />
classification <strong>of</strong> stage.<br />
AJCC/UICC Staging for Plasma Cell Myeloma<br />
Stage I<br />
Hemoglobin greater than 10.0 g/dL (100 g/L)<br />
Serum calcium 12 mg/dL or less (3.0 mmol/L)<br />
Normal bone x-rays or a solitary bone lesion<br />
IgG less than 5 g/dL (50 g/L)<br />
IgA less than 3 g/dL (30 g/L)<br />
Urine M-protein less than 4 g/24 hours<br />
Test US SI<br />
Hgb >10.0g/dL >100 g/L<br />
Serium calcium 12 mg/dL or less 3.0 mmol/L or less<br />
IgG < 5g/dL
Hemoglobin less than 8.5 g/dL (85 g/L)<br />
Serum calcium greater than 12 mg/dL (3.0 mmol/L)<br />
Advanced lytic bone lesions<br />
IgG greater than 7 g/dL (70 g/L)<br />
IgA greater than 5 g/dL (50 g/L)<br />
Urine M-protein greater than 12 g/24 hours<br />
Test US SI<br />
Hemoglobin 3.0 mmol/L<br />
IgG >7g/dL >70 g/L<br />
IgA >5 g/dL >50 g/L<br />
Stage II<br />
Disease fitting neither stage I nor stage III<br />
Note: Patients are further classified as (A) serum creatinine less than 2.0 mg/dL (177 mmol/L),<br />
or (B) serum creatinine 2.0 mg/dL (177 mmol/L) or greater. The median survival for stage IA<br />
disease is about 5 years, and that for stage IIIB disease is 15 months. These predicted<br />
survivals, however, may underestimate expected survival for these patient populations with<br />
modern therapy.<br />
B. Aspiration/Biopsy Site<br />
Bone marrow sampling (aspiration and trephine core biopsy) is usually performed at the<br />
posterior iliac crest. 3 Aspirations and biopsies may be unilateral or bilateral, depending on the<br />
indication for the bone marrow biopsy as well as clinician preference. Rarely, a sternal<br />
aspiration may be performed if only a bone marrow aspirate specimen is necessary. Sternal<br />
aspirations should only be considered as a last resort and should be performed only by persons<br />
with extensive experience with this procedure. Occasionally, the anterior iliac crest or tibia may<br />
be the site <strong>of</strong> the biopsy, depending on patient age and other unique characteristics <strong>of</strong> the<br />
patient.<br />
C. Histologic Type<br />
This protocol recommends assigning histologic type based on the WHO classification <strong>of</strong><br />
lymphoid neoplasms. 1 Originally published in 2001 and revised and updated in 2008, this<br />
classification incorporates the morphologic, immunophenotypic, cytogenetic, and molecular<br />
findings into the final diagnosis. Whereas histologic examination remains <strong>of</strong> paramount<br />
importance, many neoplasms will require the use <strong>of</strong> these ancillary studies to arrive at the<br />
correct diagnosis. 10-15 It may not be possible to provide a specific lymphoma diagnosis with<br />
bone marrow examination, particularly for patients in whom the bone marrow is the first<br />
identified site <strong>of</strong> involvement. Some <strong>of</strong> the entities provided in the case summary may be<br />
extremely uncommon in the bone marrow or may have not been described but are listed for<br />
completeness.<br />
D. Cytochemical/Special Stains<br />
Numerous cytochemical or special stains may be utilized in the diagnosis <strong>of</strong> hematopoietic<br />
neoplasms involving the bone marrow. 3 An iron (Prussian blue) stain is paramount in the<br />
evaluation <strong>of</strong> myelodysplastic syndromes and some myeloproliferative disorders. A reticulin<br />
stain is necessary for the diagnosis <strong>of</strong> some myeloproliferative disorders but may be valuable in<br />
evaluation <strong>of</strong> numerous other disorders such as hairy cell leukemia. Cytochemical stain for
myeloperoxidase is rapid, convenient, and helpful for the assessment <strong>of</strong> myeloid neoplasms.<br />
Cytochemical stains for leukocyte alkaline phosphatase, and naphthol-ASD chloroacetate<br />
esterase provide information related to cell origin or specific disease states. Cytochemical<br />
stains, however, are no longer required for the diagnosis <strong>of</strong> most disorders.<br />
E. Immunophenotyping by Flow Cytometry and/or<br />
Immunohistochemistry<br />
Immunophenotyping <strong>of</strong> bone marrow specimens can be performed by flow cytometry 10 or<br />
immunohistochemistry. 11 Each has advantages and disadvantages. Flow cytometry is rapid<br />
(hours), quantitative, and allows multiple antigens to be evaluated on the same cell<br />
simultaneously. Flow cytometry may also allow for the detection <strong>of</strong> minimal residual disease,<br />
especially in situations in which there is a unique expression pattern. Antigen reactivity,<br />
however, cannot be correlated with architecture or cytologic features. In patients from whom a<br />
dry tap is obtained, an additional bone marrow core biopsy submitted fresh in transport medium<br />
can be disaggregated and utilized for flow cytometry immunophenotyping.<br />
Immunohistochemistry requires hours/days to perform, quantitation is subjective, but importantly<br />
it allows correlation <strong>of</strong> antigen expression with architecture and cytology. Not all antibodies are<br />
available for immunohistochemistry, particularly in fixed tissues, but one <strong>of</strong> its advantages is that<br />
it can be performed on archival tissue. Both techniques can provide diagnostic, prognostic, and<br />
therapeutic information. Documentation <strong>of</strong> expression <strong>of</strong> antigens such as CD20, CD33, and<br />
CD52 by the neoplastic population can aid the clinician in selection <strong>of</strong> potential therapeutic<br />
options such as monoclonal antibody therapy. The specific immunophenotypes for individual<br />
hematopoietic disorders involving the bone marrow are readily available within the WHO<br />
Classification <strong>of</strong> Tumours <strong>of</strong> Haematopoietic and Lymphoid Tissues as well as many other<br />
hematopathology textbooks. 1,3,5<br />
F. Cytogenetic and Molecular Genetic Studies<br />
Within the WHO classification <strong>of</strong> hematopoietic disorders, significant emphasis has been placed<br />
on cytogenetic and molecular genetic studies. More than ever before, specific cytogenetic<br />
findings are helpful for the diagnosis <strong>of</strong> specific neoplasms and disease states. 12-16 In fact,<br />
many acute leukemias are currently defined based upon their specific cytogenetic<br />
abnormalities. 1 Given the importance now placed upon knowing the cytogenetic or molecular<br />
results (as determined by karyotyping, FISH, or PCR results) it is <strong>of</strong> paramount importance that<br />
care is taken to evaluate the need for these studies at the time <strong>of</strong> biopsy. FISH studies can be<br />
also performed on air-dried unfixed slides, and in some cases DNA can be scraped <strong>of</strong>f air-dried<br />
unfixed slides for molecular studies. Since karyotyping requires growing viable cells in culture, it<br />
is necessary to submit fresh specimens promptly to help ensure the best opportunity for a<br />
successful study.<br />
References<br />
1. Swerdlow S, Campo E, Harris N, Jaffe E, et al, eds. WHO Classification <strong>of</strong> Tumours <strong>of</strong><br />
Haematopoietic and Lymphoid Tissues. Geneva, Switzerland: WHO Press; 2008.<br />
2. Hussong JW, Arber DA, Bradley KT, et al. Protocol for the examination <strong>of</strong> specimens<br />
from patients with Hodgkin lymphoma. In: Reporting on Cancer Specimens: Case<br />
Summaries and Background Documentation. Northfield, IL: College <strong>of</strong> American<br />
Pathologists; 2009.<br />
3. Foucar K, ed. Bone Marrow Pathology. Chicago, IL: ASCP Press; 2001.<br />
4. Zhang Q, Foucar K. Bone marrow involvement by Hodgkin and non-Hodgkin<br />
lymphomas. Hematol Oncol Clin North Am. 2009;23(4):873-902.<br />
5. Hsi E, Goldblum J, eds. <strong>Hematopathology</strong>. Philadelphia, PA: Churchill Livingstone<br />
Elsevier; 2007.
6. Lymphoid neoplasms. In: Edge SB, Byrd DR, Carducci MA, Compton CC, eds. AJCC<br />
Cancer Staging <strong>Manual</strong>. 7 th ed. New York, NY: Springer; 2009.<br />
7. Sobin LH, Gospodarowicz M, Wittekind Ch, eds. UICC TNM Classification <strong>of</strong> Malignant<br />
Tumours. 7th ed. New York, NY: Wiley-Liss; 2009.<br />
8. Durie B, Salmon S. A clinical staging system for multiple myeloma: correlation <strong>of</strong><br />
measured myeloma mass with presenting clinical features, response to treatment, and<br />
survival. Cancer. 1975;36(3):842-854.<br />
9. Cairo et al. Non-Hodgkin’s lymphoma in children. In: Kufe P, Weishelbuam R, et al, eds.<br />
Cancer Medicine. 7 th ed. London: BC Decker; 2006:1962-1975.<br />
10. Craig F, Foon K. Flow cytometric immunophenotyping for hematologic neoplasms.<br />
Blood. 2008;111(8):3941-3967.<br />
11. Jaffe E, Banks P, Nathwani B, et al. Recommendations for the reporting <strong>of</strong> lymphoid<br />
neoplasms: a report from the Association <strong>of</strong> Directors <strong>of</strong> Anatomic and Surgical<br />
Pathology. Mod Pathol. 2004;17(1):131-135.<br />
12. Bagg A. Molecular diagnosis in lymphomas. Curr Oncol Rep. 2004;6(5):369-379.<br />
13. Merker J, Arber D. Molecular diagnostics <strong>of</strong> non-Hodgkin lymphoma. Expert Opin Med<br />
Diagn. 2007;1(1):47-63.<br />
14. Bagg A. Role <strong>of</strong> molecular studies in the classification <strong>of</strong> lymphoma. Expert Rev Mol<br />
Diagn. 2004;4(1):83-97.<br />
15. Sen F, Vega F, Medeiros LJ. Molecular genetic methods in the diagnosis <strong>of</strong> hematologic<br />
neoplasms. Semin Diagn Pathol. 2002;19(2):72-93.<br />
16. Weinberg O, Seetharam M, et al. Clinical characterization <strong>of</strong> acute myeloid leukemia<br />
with myelodysplastic-related changes as defined by the 2008 WHO classification<br />
system. Blood. 2009;113(9):1906-1908.
Bone Marrow Laboratory<br />
Test Specifications
TEST SPECIFICATIONS: BONE MARROW LABORATORY<br />
Bone Marrow Aspirate Smears, Bone Marrow Particle Preparation, and<br />
Bone Marrow Biopsy<br />
DELIVER ALL SAMPLES DIRECTLY TO THE FLOW CYTOMETRY LABORATORY<br />
CLB<br />
Room 9032<br />
3477 Euler Way<br />
Pittsburgh, PA 15213<br />
Phone: 412-864-6173<br />
Fax: 412-864-6102<br />
BONE MARROW LABORATORY HOURS OF OPERATION<br />
Monday through Friday: 8:00am to 5:00pm. The bone marrow specimens can be sent anytime. However<br />
they will be processed the following working day if received after hours <strong>of</strong> operation. The laboratory is<br />
also closed on the following holidays: New Years Day, Martin Luther King Day, Memorial Day, July 4 th ,<br />
Labor Day, Thanksgiving Day, and Christmas Day.<br />
SPECIAL INSTRUCTIONS<br />
Send a completed requisition.<br />
Call the Clinical Flow Cytometry Laboratory (412-864-6173) first before sending, to make prior<br />
arrangements about where to deliver.<br />
In packaging bone marrows, please:<br />
1. Tightly close the biopsy container. Keep container upright.<br />
2. MARK THE DATE AND TIME THE BIOPSY WAS PLACED IN THE B-PLUS FIXATIVE – on the<br />
container.<br />
3. Package slides separately from the biopsy specimen (to avoid vapor fixation <strong>of</strong> the slides).<br />
Use adequate safety measures when transporting specimens.<br />
BONE MARROW EXAMINATION TEST DESCRIPTIONS<br />
Bone marrow examinations may include aspiration with smears, particle preparations or clot sections and<br />
biopsies. Aspirate smears are most useful to examine the cytological detail <strong>of</strong> the marrow elements and<br />
for cytochemical and immunocytochemical stains. The aspirated material may also be used for culture,<br />
flow cytometric immunophenotyping, cytogenetic, molecular, and other special studies.<br />
The particle preparation represents non-decalcified tissue sections <strong>of</strong> filtered bone marrow aspirate<br />
spicules. This allows for histopathologic analysis <strong>of</strong> a large volume <strong>of</strong> marrow with excellent morphology<br />
and an intact architecture so features such as cellularity can be easily assessed. It is suitable for a wide<br />
range <strong>of</strong> special stains including immunohistologic stains.<br />
Bone marrow core biopsies are decalcified, sectioned, and routinely stained with H&E and PAS stains.<br />
Biopsy specimens are used to evaluate cellularity, hematopoiesis, non-aspirable lesions to see the<br />
topographical relationship <strong>of</strong> focal lesions to the bony trabeculae, and for evaluation <strong>of</strong> bone and vessels.<br />
BONE MARROW ASPIRATE SMEAR SPECIMEN REQUIREMENTS<br />
10-20 bone marrow slides should be made at the bedside from the first syringe pulled. Label each<br />
slide with name, date, and time <strong>of</strong> collection. The number <strong>of</strong> slides sent will vary depending on the<br />
tests requested. Send the slides in either durable cardboard slide holders or plastic slide holders<br />
accompanied by a requisition.<br />
If bilateral (right and left hips) bone marrows are done, then label slides left or right hip.<br />
Send the slides in either durable cardboard slide holders or plastic slide holders. The number <strong>of</strong><br />
slides sent will vary depending on the test requested.<br />
For a basic bone marrow aspirate interpretation based on Wright-Giemsa stains, send two labeled<br />
slides plus a recent CBC/differential result and the corresponding peripheral blood smear.
If cytochemical or iron stains are requested, the following is a list <strong>of</strong> the minimum required number <strong>of</strong><br />
unstained slides that should be sent. Send one additional slide for a Wright-Giemsa stain in all cases.<br />
In addition, extra unstained slides are useful if any stains need to be repeated.<br />
1 SLIDE:<br />
Iron Stain<br />
Naphthyl AS-D Chloroacetate esterase stain (CAE)<br />
Myeloperoxidase cytochemical stain (MPO)<br />
Sudan Black Stain<br />
Periodic Acid Schiff Stain (PAS)<br />
2 SLIDES:<br />
Non-specific Alpha Naphthol Acetate Esterase Stain (NSE)<br />
Double Esterase Stain (NSE+CAE)<br />
If there are any questions regarding cytochemical stains call the Special Testing Laboratory at<br />
(412) 864-6179.<br />
BONE MARROW PARTICLE PREPARATION SPECIMEN REQUIREMENTS<br />
Five milliliters <strong>of</strong> bone marrow aspirate (preferably from the first or second syringe), placed in a yellow<br />
top (ACD) Vacutainer tube.<br />
Store at room temperature.<br />
The material for the particle preparation will be filtered, fixed, and processed by the bone marrow and<br />
histology laboratories, at UPMC Oakland.<br />
In addition submit two aspirate smears, the most recent CBC/differential and corresponding<br />
peripheral blood smear.<br />
BONE MARROW BIOPSY SPECIMEN REQUIREMENTS<br />
4-6 touch preparations <strong>of</strong> the biopsy specimen (3 imprints on each slide) made at the bedside. Label<br />
each slide with patient's name, date, and time <strong>of</strong> collection.<br />
Place the biopsy into B-Plus fixative. MARK THE TIME AND DATE THE BIOPSY WAS PLACED IN<br />
THE B-PLUS FIXATIVE ON THE CONTAINER. The B-Plus fixed biopsy should be delivered to the<br />
laboratory the same day it is done. Prolonged fixation in B-Plus Fixative makes the biopsy difficult to<br />
process.<br />
If performing bilateral (right and left hips) biopsies, each biopsy must be placed in SEPARATE B-Plus<br />
fixative containers and labeled left or right hip.<br />
In addition submit two aspirate smears, the most recent CBC/differential and corresponding<br />
peripheral blood smear.<br />
WARNING: B-Plus fixative contains formaldehyde. Formaldehyde is a carcinogen, irritant, and<br />
highly toxic. Avoid contact with eyes, skin, and clothing. Do not inhale.<br />
CONTACTS, DIVISION OF HEMATOPATHOLOGY<br />
Steven Swerdlow, M.D. Director, Division <strong>of</strong> <strong>Hematopathology</strong> (412) 647-5191<br />
Miroslav Djokic, M.D. Director, Adult Bone Marrow Service (412) 692-2128<br />
Celina Fortunato Lead technologist, Bone Marrow Lab (412) 647-0263<br />
Rev 4/13
Summary <strong>of</strong> Test Specifications for ancillary laboratories (principally for<br />
marrows)<br />
*Flow cytometry (see also detailed test specifications)<br />
-Bone marrow aspirate (2-4 ml) is placed into a heparinized Vacutainer.<br />
Cytogenetics (chromosome analysis)<br />
-Bone marrow aspirate (1 ml) is placed into thawed cytogenetics media. A<br />
heparinized (green top) Vacutainer can be used if there is no media available.<br />
*Gene rearrangement (molecular oncology)<br />
-Bone marrow aspirate (2.5 ml) is placed into an EDTA Vacutainer.<br />
*Cultures<br />
-For bacterial cultures fill 3 small isolator tubes (3-cc total) with bone marrow<br />
aspirate.<br />
-For viral cultures place 1-2 ml <strong>of</strong> bone marrow aspirate into a heparinized<br />
Vacutainer. (Excluding Parvovirus)<br />
*Parvovirus by PCR<br />
-Place bone marrow aspirate (2.5 ml) into an EDTA Vacutainer.<br />
-This test is sent to Magee Hospital.<br />
*CMV by PCR<br />
-Place bone marrow aspirates (1-5 ml) into ACD Vacutainer.<br />
-Test is performed in virology lab.
Bone Marrow After-Hours<br />
Procedures
AFTER-HOURS PROCEDURES (CHILDREN’S & ADULT BONE MARROWS)<br />
1. The Bone marrow technologist is available Monday through Friday between the hours <strong>of</strong><br />
8:00 AM and 4:00 PM. (exclusive <strong>of</strong> weekends and all UPMC holidays). At all other<br />
times (except on the night shift), a technologist from the Automated Testing Lab (ATL)<br />
will be available to assist with the bone marrow procedure. For assistance at UPMC<br />
Presbyterian, call (412) 647-6199. For assistance at UPMC Shadyside, call (412) 623-<br />
6011. For assistance at UPMC CHP Lawrenceville, call (412) 864-9877.<br />
2. Procedures to follow at UPMC- PUH are located in the Adult Bone Marrow Procedure<br />
<strong>Manual</strong> (room G325.1) and in the Bone Marrow Procedure <strong>Manual</strong> <strong>of</strong> the Hematology<br />
Procedure <strong>Manual</strong> (Automated Testing Lab).<br />
3. For those weekend bone marrows requiring immediate attention (e.g. acute leukemia)<br />
performed at UPMC-Presbyterian or CHP, the ATL technologist will triage the sample<br />
for ancillary tests, perform a quick stain <strong>of</strong> the aspirate smears and notify the ‘on call’<br />
Clinical Pathology (CP) resident and hematopathologist.<br />
4. Bone marrow specimen processing:<br />
Aspirate smears (ADULTS):<br />
Emergent cases: Two aspirate smears are stained in the Automated Testing<br />
Laboratory and placed in a folder with requisitions, current peripheral blood slide and<br />
current CBC and differential, for pathologist to review. After pathologist review, place<br />
all material in the G325.1 Bone Marrow Reading room.<br />
Non-emergent cases: Aspirate smears are left in the Automated Testing<br />
Laboratory and sent to the Bone marrow processing area in 9032 CLB the following<br />
working day.<br />
Aspirate smears (CHP):<br />
The CHP hematology technologist is available Monday through Sunday between the<br />
hours <strong>of</strong> 7 a.m. and 8 p.m. (inclusive all UPMC holidays). CALL 412-864-9877<br />
The technologist will assist with the smears, triage the sample for ancillary tests,<br />
perform a quick stain, and notify the “on call” pathology resident and<br />
hematopathologist.<br />
CHP Aspirate smears on Saturday, Sunday, holidays, and in emergency<br />
situations:<br />
Two aspirate smears are stained at CHP laboratory. One is placed in a folder in the<br />
CHP Heme lab for the Heme/Onc clinician to review. The second BM aspirate, a<br />
current stained peripheral blood slide, current CBC results and a copy <strong>of</strong> the<br />
<strong>Hematopathology</strong> requisition are sent to the PUH ATL laboratory 5 th floor CLB, room<br />
5011 to the attention <strong>of</strong> the lead technologist. The CP resident on call and<br />
hematopathologist should be paged and notified <strong>of</strong> the case by the CHP<br />
technologist. The PUH Heme lead technologists will be notified by telephone. The<br />
PUH lead technologist will page the pathologist and chief pathology resident when<br />
the specimen arrives. The rest <strong>of</strong> the bone marrow aspirate smears should be<br />
placed in the plastic containers (after drying) and together with the biopsy sent to the<br />
Flow Cytometry lab, CLB 9032.
Bone Marrow biopsy on cases from UPMC-Presbyterian /Shadyside, UPMC<br />
CHP or outside:<br />
<br />
<br />
<br />
Monday – Thursday evenings: All CHP biopsies designated for hematopathology, all<br />
PUH and SHY adult biopsies are left in the Hematology labs <strong>of</strong> respective hospitals.<br />
Monday - Friday evenings all outside bone marrow cases are delivered to the Flow<br />
Cytometry area (9032 CLB).<br />
Friday evening after 5:30 pm – Monday morning: the biopsy is kept with the rest <strong>of</strong><br />
the case the specimen processing room, CLB 9032. This procedure is also followed<br />
if Monday is a holiday.<br />
Emergent bone marrow biopsies on weekends and holidays:<br />
After the Hematopathologist’s approval, the resident on call will make the biopsy<br />
Same Day Rush Priority. The resident will pick up the bone marrow biopsy from<br />
9032 CLB processing area, indicate this on top <strong>of</strong> the requisition that is left with the<br />
rest <strong>of</strong> the specimen in CLB, take biopsy to the Gross room for PA to accession and<br />
gross in. The gross room PA is only available on Saturdays from 8AM-2PM. (For<br />
accessioning and grossing directions <strong>of</strong> bone marrows see AP procedure manual<br />
located in the PUH Gross Room on 6A or Special Hematology Processing <strong>Manual</strong><br />
located in 9032 CLB). <strong>Resident</strong> on call will contact Histotechnologist on call (pager<br />
12239) about the bone marrow biopsy and take the specimen to the Histology lab.<br />
Histotechnologist will process the specimen on the overnight processor so the<br />
specimen will be ready the next working day.<br />
5. Flow Cytometry:<br />
If specimens cannot be delivered by 5:30 PM Monday through Thursday, leave the<br />
specimen at room temperature at the PUH or CHP Hematology lab. It will be sent to<br />
the Flow Cytometry lab the next working day.<br />
If specimens cannot be delivered by 5:30 PM on Friday, or if there is a specimen<br />
obtained before noon on Saturday, call the Flow Cytometry lab between 8 AM and 1<br />
PM on Saturday at 412-864-6173 to inform them that a specimen is being sent to the<br />
Flow Cytometry lab. Leave an Audix message if the Flow lab is closed.<br />
Specimens from Saturday afternoon, Sunday, Thanksgiving, Christmas and other<br />
holidays after 1 PM, should be held at room temperature and then sent to the Flow<br />
Cytometry lab the following normal working day.<br />
Any holiday that falls on Monday: flow technologist is on call (pager 412-565-9563)<br />
between 9-1 PM for the hematopathologist on call.<br />
Any holiday that falls on a weekday: flow lab is usually closed. However, flow lab is<br />
never closed 2 days in a row. Check with attending if a major holiday is on weekend.<br />
In an emergency on all other Monday holidays before 1:00 PM, clinicians are to<br />
page the clinical pathology resident on-call who will then contact the<br />
hematopathologist on call.
6. Cytogenetics:<br />
Monday through Thursday, specimens that cannot be received by the Cytogenetics<br />
lab before 5:30 PM should be left at room temperature overnight. Specimens are<br />
sent to the Cytogenetics lab the next day.<br />
For specimens obtained Fridays that cannot be received by the Cytogenetics lab by<br />
5:30 PM, leave the specimen at room temperature in the Cytogenetics bin in the<br />
PUH-ATL specimen processing area or CHP-Hematology area for pick up on<br />
Saturday.<br />
Saturday/Sunday/Holiday specimens: Be sure that the specimen gets to the PUH-<br />
ATL specimen processor. The CHP specimen will be delivered to the Cytogenetics<br />
lab directly from CHP hematology. The PUH-ATL specimen processor or CHP<br />
technologists will contact the Cytogenetics lab by page at (412) 917-9458.<br />
Cytogenetic lab personnel arrange for the courier pick up <strong>of</strong> these specimens.<br />
Alternatively, call (412) 641-6688 and follow Audix instructions.<br />
7. Molecular Diagnostic studies:<br />
Specimens should get to the lab by 4 PM if at all possible. If other arrangements<br />
need to be made or any questions answered, the attending on-call will be available<br />
at pager (412) 433-9591. They may be able to have someone come in on Saturday.<br />
Specimens that have to be held over the weekend or holidays are held as follows:<br />
o DNA testing: Keep the samples at room<br />
temperature.<br />
o RNA testing: Refrigerate the samples at 4 o C.<br />
o DNA and RNA testing: Refrigerate the samples at 4 o C.<br />
o Tissue samples: Freeze the samples at -20 o C.<br />
8. The Flow Cytometry Lab, processing room 9032 CLB is the central receiving area for<br />
bone marrow specimens received from UPMC and non-UPMC affiliated hospitals. The<br />
lab is open from 8:00 AM to 6:00 PM, Monday through Friday and from 8:00 AM to 4:30<br />
PM Saturday. During these hours the flow technologists/processors are responsible for<br />
triaging the specimens they receive (except as noted in numbers 3 and 4). If they<br />
receive a marrow requiring emergency review after 5 p.m. or on the weekend, they will<br />
forward the appropriate material to the ATL lab to the attention <strong>of</strong> the hematology lead<br />
technologist who will follow the after hour procedures detailed in 4. Any cases that<br />
require triaging or review outside <strong>of</strong> these hours should be sent to the UPMC-<br />
Presbyterian ATL lab to the attention <strong>of</strong> the hematology lead technologist.<br />
If a routine bone marrow specimen is delivered to the PUH Gross Room, hold specimen<br />
to the next working day and either send to station #390 or via Medspeed to room 9032<br />
CLB.<br />
Revised 10/2013
Lymph Node<br />
Experience
Lymph Node Pathology Experience (~4 weeks)<br />
Lymph Node Sign Out<br />
A. “On call” for processing <strong>of</strong> fresh lymph node biopsies.<br />
NOTE: One <strong>of</strong> the lymph node assistants are available to help gross from 9-5:30/6 pm. The lymph node<br />
assistant pager number is 412-958-7432. They will independently gross most specimens but may require help<br />
either over the telephone with images that can be seen using our gross imaging “telepathology” or you may<br />
need to help them in person in the CLB (Room 9032). If you get called during these hours, be sure to page the<br />
lymph node assistant if they are not already in or on their way to the grossing area in the flow cytometry<br />
laboratory (CLB 9032). After 5:30pm, the <strong>Hematopathology</strong> fellow or resident on an extended day shift (see<br />
<strong>Hematopathology</strong> Schedule) can help. On occasion, one <strong>of</strong> the lymph node assistant should be able to gross<br />
in lymph nodes or help out until 6:00 pm.<br />
Become familiar with detailed lymph node protocol, spleen protocol and consult procedures.<br />
B. Sign out <strong>of</strong> both fresh and consult lymph nodes and related material with staff. Be sure to meet<br />
with hematopathologist on lymph node service to review organizational aspects <strong>of</strong> this rotation.<br />
Become familiar with role <strong>of</strong> the lymph node assistant, use <strong>of</strong> hematopathology case worksheet,<br />
established procedure for organization <strong>of</strong> sign-out materials and guidelines for dictating reports.<br />
C. Review <strong>of</strong> educational materials.<br />
• Checkpath (images, histories and explanations <strong>of</strong> faculty CME program for <strong>Hematopathology</strong>)<br />
(PUH G315 and in Dr. Swerdlow’s coordinator’s <strong>of</strong>fice).<br />
• Teaching/conference sets <strong>of</strong> glass slides <strong>of</strong> marrows, lymph nodes, etc. (Dr. Swerdlow’s <strong>of</strong>fice).<br />
• Lymph node chapter in Sternberg.<br />
• Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E., Pileri, S.A., Stein, H., Thiele, J., Vardiman, J.<br />
(Eds.): WHO Classification <strong>of</strong> Tumours Pathology <strong>of</strong> Haematopoietic and Lymphoid Tumours,<br />
IARC, Lyon, 2008.<br />
• Ioachim, HL Medeiros, LJ. Ioachim’s Lymph Node Pathology, 4 th edition, 2009.<br />
• Jaffe ES, Harris NL, Vardiman J, Campo E, and Arber D. <strong>Hematopathology</strong>, 2010<br />
• Leonard, D Diagnostic Molecular Pathology (Volume 41 in the series “Major Problems in<br />
Pathology”), Saunders, 2003.<br />
• Web-based resources.<br />
D. Review results <strong>of</strong> ancillary studies procedures performed on lymph nodes you have reviewed<br />
and read addenda that faculty issue.
APPENDIX I -SURGICAL PATHOLOGY MANUAL: LYMPH NODE PROTOCOL<br />
Subject: The protocol is for lymph node or extranodal biopsies with suspected hematopoietic or lymphoid<br />
disorders.<br />
1.0 Principle<br />
Diagnostic lymph node biopsies and other biopsies with suspected lymphoproliferative disorders should be<br />
handled in a standard fashion to optimize histiologic sections and make appropriate ancillary studies<br />
available.<br />
2.0 Specimen<br />
2.1 Criteria:<br />
All tissues where lymphoma is suspected or documented in the patient history as well as all lymph node<br />
biopsies in which the only specimen submitted for study is the nodal tissue, and if performed, a frozen<br />
section has excluded metastatic carcinoma. Cases where post-transplant lymphoproliferative disorders are<br />
suspected are not included unless requested by the Division <strong>of</strong> Transplant Pathology. Lymph node<br />
excisions performed solely as non-lymphoma staging procedures are not included unless gross, touch<br />
imprint or frozen section studies suggest a hematopoietic/lymphoid neoplasm. The protocol should be<br />
performed under the supervision <strong>of</strong> a hematopathologist or designate if at all possible.<br />
Emergent lymph nodes on weekends and holidays:<br />
The AP resident should be paged who will determine the most appropriate resident on call to handle the<br />
specimen. After hematopathologist’s approval, the resident on call will make the LN specimen Same Day<br />
Rush Priority. The on-call resident will contact the histotechnologist on call (pager 12239) and take the LN<br />
specimen to the Histology lab located in the CLB room 3018. The Histotechnologist on call will place the<br />
LN specimen on the overnight processor so the specimen will be ready the next working day.<br />
2.2 Fixation:<br />
Fresh or saline moistened tissue. RPMI essential media is used to transport tissue. Formalin or any other<br />
chemically fixed tissue cannot be processed using this protocol and will be handled according to general<br />
cutting manual techniques. Specimens received in formalin in which lymphoma is suspected should have<br />
at least one section post-fixed in B+ fixative.<br />
2.3 Sample size:<br />
Any size tissue can be processed.<br />
3.0 Reagents, Supplies and Equipment<br />
3.1 Reagents:<br />
B+ Fixative<br />
100% formalin<br />
10% formalin<br />
OCT
(Warning: Formalin is a suspected carcinogen. HANDLE WITH CAUTION. DO NOT INHALE. See<br />
MSDS Binder.)<br />
(Warning: B+ fixative contains zinc chloride and formaldehyde. HANDLE WITH CAUTION. DO NOT<br />
INHALE. See MSDS Binder.)<br />
3.2 Supplies:<br />
Lymph Node Gross Dictation Sheet (Appendix E)<br />
Log for freezer (Appendix C)<br />
Liquid nitrogen<br />
Cytocentrifuge cardboard<br />
Slides (Regular and “+” slides)<br />
RPMI essential media<br />
Beam capsules<br />
Marking pens<br />
Tissue processing cassettes<br />
Personal protective supplies<br />
Gloves<br />
Apron<br />
Shield<br />
Plastic freezer box to hold Beam Capsules<br />
Saline solution for Molecular studies<br />
Specimen bags<br />
Sterile containers<br />
Forms for Molecular Diagnostics (Appendix J)<br />
Forms for Cytogenetics Lab (Appendix K)<br />
3.3 Equipment:<br />
Liquid Nitrogen tank<br />
-80° degree freezer<br />
Scalpel<br />
Forceps<br />
4.0 Calibration and Standardization<br />
Not Applicable<br />
5.0 Quality Control<br />
5.1 All problems noted with specimen processing will be entered under the "Adverse Event" section for<br />
each case in CoPath. (See Appendix G.)<br />
6.0 Procedure<br />
6.1 If a frozen section is requested, call the responsible surgical pathologist and resident. The first part <strong>of</strong><br />
the gross description should be filled in on the template. The specimen should be kept as intact as
possible and cut as illustrated and described in sections 6.7-6.10. If the frozen section shows<br />
metastatic carcinoma, the case will be handled as per routine, in the surgical pathology division. If<br />
metastatic carcinoma is not identified, continue with protocol. The cassette designation for the<br />
resubmitted frozen section should be filled in on the gross description template—(FS).<br />
NOTE : ALL HEMEPATH SPECIMENS ARE GROSSED IN THE FLOW CYTOMETRY LABORATORY –<br />
CLB Room 9032<br />
6.2 Place tissue in gauze soaked in RPMI in a sealed container labeled with patient name and surgical<br />
number.<br />
6.3 Page the hematopathology LN assistant first. If LN assistant does not answer, page the<br />
hematopathology fellow or resident on call for lymph nodes (See schedule). If any problem, call the<br />
<strong>Hematopathology</strong> Division Coordinator at 647-5191 and have her contact the <strong>Hematopathology</strong> resident or<br />
fellow on call. If Coordinator does not answer, call the <strong>Hematopathology</strong> secretary 412-647-0382 with the<br />
same instructions. If the LN assistant and trainee on call or on the LN service cannot be found, page the<br />
faculty on the LN service or if necessary the faculty on call.<br />
If no one answers, contact any <strong>of</strong> the hematopathology fellows or any other hematopathology faculty.<br />
6.4 Specimens that arrive in the evening will be handled by the hematopathology fellow or resident on an<br />
extended day shift who will follow this protocol as best they can. Material may be held in RPMI for flow<br />
cytometry but this should only be done if there is enough tissue for 2 good histologic sections plus<br />
enough for 3 snap frozen pieces. No material will go directly to the molecular laboratory. If the<br />
following day is a workday and you are unfamiliar with this protocol, place the entire specimen in RPMI,<br />
place in the refrigerator and notify hematopathology the following morning (See 6.3).<br />
Emergent lymph nodes on weekends and holidays: After hematopathologist’s approval, the resident<br />
on call, assigned by the Chief resident, will make the LN specimen Same Day Rush Priority . The<br />
resident will contact the histotechnologist on call (pager 12239) and take the LN specimen to the<br />
Histology lab; CLB-2 nd Floor. The Histotechnologist on call will place the LN specimen on the overnight<br />
processor so the specimen will be ready the next working day.<br />
6.5 Inform the lymph node service assistant or resident, fellow or, if necessary, the hematopathologist on<br />
lymph node service that tissue has arrived in the Flow Cytometry Laboratory CLB Room 9032.<br />
6.6 Observe tissue grossly and write down brief gross description on template. (Appendix D) Describe<br />
size, color, and consistency.<br />
6.7 Cut lymph node perpendicular to the long axis. Keep tissue moist at all times:<br />
* *
6.8 Cut a small paper thin slice, place on a cytocentrifuge preparation cardboard and make 2 fixed imprints<br />
(done on regular slides) which can be stained immediately with H&E and 3-5 air dried imprints (done on “+”<br />
slides) to be saved at the LN grossing station until the case is completed (for additional special studies, if<br />
appropriate). Unused slides are filed in the slide file drawers. The code TP documents the test.<br />
6.8.1 Look at the touch imprint to make a preliminary assessment <strong>of</strong> the lymph node. If<br />
performing flow cytometric studies, check if any special studies should be performed (e.g., if the<br />
cells appear blastic, an acute leukemia panel should be run) and about the size <strong>of</strong> the cells that<br />
are <strong>of</strong> interest (small, large, mixed) and inform the flow technologist. If no one is present in the<br />
flow lab write the request on the requisition and place it together with the specimen in the<br />
refrigerator located at the flow lab CLB 9 th floor- Room 9032 (See 6.3).<br />
6.9 The central portion <strong>of</strong> the lymph node should be cut into 2-3 mm slices and if >2 cm in maximum<br />
dimension, hemisected (sometimes it is easier to initially cut 5-6 mm slices and after brief fixation to slice in<br />
half). Include the node capsule in the sections. Be sure to include any focal lesions. No pieces should be<br />
larger than 1.5 x 2 cm.<br />
6.9.1 Fix at least one section in 10% neutral buffered formalin. If tissue is very limited, formalin<br />
fixation takes precedence over B+ fixation.<br />
6.9.2 Each cassette has a unique designation (ACMB/DNA, BB+, C, etc). Be sure to use the<br />
appropriate cassettes. PH White Rule out lymphoma cassettes will be labeled using the<br />
cassette engraver with the surgical pathology number and a unique block designation<br />
[A(CMB/DNA) OR AFS (for frozen section), BB+, C, etc.] by selecting PH White (R/O<br />
Lymphoma). For needle core biopsies or tiny pieces <strong>of</strong> tissue, specimens should be marked<br />
with eosin and wrapped in filter paper and put into mesh cassettes. Note in your gross<br />
description if you feel the tissue may not survive processing.<br />
6.10 Use end positions (*) for the following (unless focal lesions are present only in<br />
this area or only in the midportion).<br />
6.10.1 Culture, if appropriate, and if the surgeon has failed to do cultures (unless sterile, only<br />
AFB and fungal cultures are generally meaningful).<br />
6.10.2 Cell suspension immunophenotypic studies (flow lab 624-3746). Keep ½ to 1 cm 3 fresh<br />
and moist for this. Place in container with 50 ml RPMI media. Label and place in a biohazard<br />
bag with a copy <strong>of</strong> the requisition with flow panel decided.<br />
6.10.3 Snap freeze tissue 3+ blocks (approximately 7x4x3 mm) in cryovial capsules (2 without<br />
OCT and 1 with OCT) labeled with the surgical pathology number in the liquid nitrogen tank in<br />
flow lab. If a surgical pathology number is unavailable at the time, print the patient’s full name<br />
and date on the tube. Snap capsules onto cryocane, dip into liquid nitrogen tank for a least 60<br />
seconds. These are to be placed in the –80 o C freezer (place in current capsule box) located in
the CLB room 9032 lymph node grossing area in the designated box and logged in on the log<br />
sheet located on the freezer.<br />
6.10.4 Submit a 7x7x5-mm piece to the Clinical Molecular Diagnostics Laboratory for possible<br />
molecular diagnostic studies. Place tissue in a container with saline labeled with surgical<br />
number and place in a self-sealing biohazard bag. Submit an adjacent piece for routine<br />
processing labeled CMB/DNA to document the nature <strong>of</strong> piece sent for molecular studies.<br />
Place a copy <strong>of</strong> the requisition and a filled out Molecular Diagnostics form in the side pouch <strong>of</strong><br />
the bag. Place a Molecular Diagnostics sticker on specimen bag. During working hours<br />
(Monday-Friday 7am-3: 45pm) tube to station #370. After hours place in lymph node fridge next<br />
to grossing bench.<br />
6.10.4.1 After hours place specimen in the lymph node refrigerator next to the grossing station on<br />
side door and send it the following day.<br />
6.10.6 If there is a high suspicion <strong>of</strong> lymphoma and enough tissue after all <strong>of</strong> the above portions<br />
are taken, place a 7x7x5 mm (or larger) fresh STERILE piece <strong>of</strong> tissue in RPMI to send to the<br />
Cytogenetic Laboratory for chromosome (karyotype) analysis. Place a Cytogenetics sticker on<br />
specimen bag.<br />
6.10.6.1 The specimen can be tubed to station #417 (Magee<br />
Womens Automated Testing Laboratory) at any time Monday-<br />
Saturday (up until 1:00pm)<br />
6.10.6.2 After 1:00pm on Saturday, place specimen in the lymph node refrigerator<br />
next to the grossing station for it to be tubed to station #417 the following<br />
Monday by the LN assistant.<br />
6.11 Note:<br />
6.11.1 A well-fixed section is the highest priority although almost no node is too small to preclude snap<br />
freezing one piece.<br />
6.11.2 Lymph nodes received in formalin can still be post-fixed in B+<br />
6.12 Once the protocol is completed, the fixed portions are submitted to histology and the completed<br />
gross description template dictated.<br />
6.13 Summary <strong>of</strong> Lymph Node Protocol<br />
6.13.1 Touch Imprints<br />
6.13.1.1 Quick fix in alcohol and stain with H&E for immediate review<br />
6.13.1.2 Air dry, place in box next to the grossing station.
6.13.2 Fresh tissue for special labs<br />
6.13.2.1 Fresh piece in RPMI for flow lab (give to the flow tech)<br />
6.13.2.2 Fresh piece in saline for molecular diagnostics (See 6.13.4.4)<br />
6.13.2.3 Fresh piece in RPMI for Cytogenetics<br />
6.13.2.4 Fresh piece to Microbiology if required.<br />
6.13.3 Snap freeze 2 pieces <strong>of</strong> fresh tissue in beem capsules and 1 piece in Beem capsule in<br />
OCT and store in box in -80 freezer located in the LN grossing area. See protocol for details.<br />
6.13.4 Fixed tissue sections<br />
6.13.4.1 If frozen section was performed, submit FS piece in cassette A in<br />
formalin.<br />
6.13.4.2 Cut thin and fix ≥ 1 sections in cassette BB+ after B+ fixative. Put time on<br />
jar.<br />
6.13.4.3 Submit one section <strong>of</strong> tissue in cassette ACMB/DNA fixed in<br />
formalin that is adjacent to the molecular diagnostics piece.<br />
6.13.4.4 Submit the remainder <strong>of</strong> tissue in cassettes C, D, E, etc.<br />
6.13.4.5 Use White colored cassettes to be engraved by selecting PH White (Rule out<br />
lymphoma) under ‘Block Detail’ in the Histology section.<br />
6.13.5 Comments<br />
6.13.5.1 If during normal working hours the lymph node assistant/<br />
hematopathology fellow/resident (staff) should be called to perform this protocol.<br />
6.13.5.2 If very little tissue is present, good histologic sections and one snap frozen piece are<br />
generally the highest priorities.<br />
6.13.5.3 A gross dictation template should be used.
6.13.6 Diagram <strong>of</strong> Summary<br />
B+ and formalin fixed sections<br />
<br />
<br />
<br />
<br />
Snap freeze 3-5 pieces<br />
Molecular Lab with<br />
adjacent piece submitted<br />
in fixative “CMB/DNA<br />
If required<br />
--Culture<br />
Cytogenetics<br />
Flow Laboratory<br />
7.0 Calculations<br />
Not Applicable<br />
<br />
<br />
Touch Preps<br />
Fix and stain<br />
Air dried & Save<br />
8.0 Reporting Results / Normal Values<br />
Not Applicable<br />
9.0 Periodic Maintenance<br />
Not Applicable<br />
10.0 Procedural and QA Notes<br />
Not Applicable<br />
11.0 Limitations<br />
Not Applicable<br />
12.0 References<br />
Banks, PM Technical Aspects <strong>of</strong> Specimen Preparation and Introduction to Special Studies. In: Jaffe, ES<br />
Surgical Pathology <strong>of</strong> the Lymph Nodes and Related Organs. pages 1-22, 1995, W.B. Saunders,<br />
Philadelphia.<br />
Leonard, D Diagnostic Molecular Pathology (Volume 41 in the series “Major Problems in Pathology”),<br />
Saunders, 2003.<br />
Revised 7/2004 LF<br />
Revised 1/2010<br />
Revised 3/2010 CF<br />
Revised 10/2013 LF
How to handle very small specimens being submitted for histology*<br />
1. Specimens should be marked with eosin and wrapped in filter paper and put into mesh<br />
cassettes. Note in your gross description if you feel the tissue may not survive processing.<br />
2. The histology lab will contact the responsible house-staff <strong>of</strong>ficer and faculty member<br />
immediately if there is a failure to identify tissue in a cassette.<br />
RECUTS OF SMALL PIECES OF TISSUE<br />
Suggestions from Dr. Yousem when you are asking for recuts <strong>of</strong> very small pieces <strong>of</strong> tissue. It would be very<br />
worthwhile to alert Histology that the fragments <strong>of</strong> tissue are small and care must be taken. Instructions in the<br />
stain comment fields are helpful.<br />
1. Tell the lab to "take sections right <strong>of</strong>f the top", "do not face the block"- this alerts them not to aggressively<br />
"face" the block<br />
2. Tell the lab to take "serial sections"-- this way they do not lose sections between the ones obtained for your<br />
stain requests<br />
3. Ask for unstained slides in addition to the required ones-- this allows you to have extras should the stain<br />
not work or tissue fall <strong>of</strong>f the slide<br />
4. If it is a very tough case, ask that an experienced technician handle the case- make this request in the<br />
comment or call the lab<br />
5. Cell blocks in cytology should always contain this type <strong>of</strong> request, in my opinion.<br />
Pictorial Version <strong>of</strong> Lymph Node Protocol<br />
(Double click on the link below for the PowerPoint Presentation)<br />
Z:\Linda Koerbel\<strong>Fellow</strong> LN grossing presentation\Grossing Fresh Lymph<br />
Nodes - LF SHS revision 12 09 updated 02-16-2012.ppt
Submitting Histology After Grossing<br />
When we receive fresh tissue (in saline or RPMI): Submit the cassettes in formalin<br />
filled container appropriately labeled “HISTOLOGY LAB” and they can either be tubed<br />
to station #320 (Histology lab) or if after hours leave on LN grossing bench to be sent<br />
the following day.<br />
NOTE- PLEASE USE YOUR JUDGEMENT
Surgical Pathology <strong>Manual</strong>: Spleen Protocol<br />
PROCEDURE:<br />
1. Measure the spleen in three dimensions and weigh.<br />
2. Section the spleen transversely at .5 to 1.0-cm intervals. *Photograph any lacerations and/or<br />
surgical tears before transverse sectioning. To take a picture with Nikon digital grossing camera, make<br />
sure all power is turned on as follows: turn on power switch on box next to the PC, turn on power button on<br />
box at camera, and turn on power switches for both lights. ’Place specimen on camera base on table. In<br />
Copath under PHS case no. using Accession Entry Edit or Image entry edit, click on Image gallery,<br />
then click “Import from TWAIN”, and then click on “C” to capture the image.<br />
3. The lymph node protocol for handling lymphomas should be followed in cases <strong>of</strong> suspected<br />
lymphoma (staging, etc.) and other hematopoietic disorders.<br />
4. Any hilar nodes or accessory spleen should be submitted separately.<br />
5. Photograph one section <strong>of</strong> spleen.<br />
DESCRIPTION:<br />
1. Weight and dimensions.<br />
2. Hilum: nature <strong>of</strong> vessels, presence <strong>of</strong> lymph nodes or accessory spleen.<br />
3. Capsule: color, thickness, focal changes, adhesions, lacerations (location, length and depth).<br />
4. Cut surfaces: color, consistency, malpighian corpuscles (size, color conspicuous), fibrous<br />
trabeculae, nodules or masses, diffuse infiltration, red or white pulp disease.<br />
SECTIONS:<br />
1. If no gross abnormality is seen, and the spleen is taken out as an incidental splenectomy, submit<br />
two to three random sections.<br />
2. If the spleen is grossly lacerated, submit two to three random sections including the area <strong>of</strong><br />
laceration.<br />
3. If it is a leukemia or lymphoma case, submit a minimum <strong>of</strong> five sections (B+ and formalin fixed),<br />
including any distinct nodules. Several nodules should be submitted in cases <strong>of</strong> focal disease. At<br />
least one section should include the capsule. Sections should be thin and no larger than 1.5 x 2.0<br />
cm.<br />
ANCILLARY STUDIES:<br />
1. Make certain that if a hematopoietic/lymphoid neoplasm is suspected, tissue is processed for all the<br />
potential ancillary studies described for lymph node biopsies.<br />
Revised 10/2013
LYMPH NODE GROSS DICTATION –TEMPLATE<br />
Dictation phone # 802-6706 Work type #9004 For multiple parts use other side>>><br />
CASE# PHS___: __________________<br />
Protocol to order in<br />
NAME: ______________________________<br />
histology- ‘Rolym’ (select<br />
MEDICAL RECORD #___________________<br />
the ‘load’ box then ‘run<br />
protocol’ button)<br />
PLEASE DICTATE CLINICAL HISTORY AS STATED ON REQUISITION<br />
The specimen is received [fresh in RPMI or formalin] labeled with the patient’s name, initials ___, MRN,<br />
and [specimen site] ”___________________” It consists<br />
<strong>of</strong>______________________________________________________________________________________<br />
________________________________________________________________________________________<br />
________________________________________________________________________________________<br />
Air dried touch preparations are performed, material is snap frozen (__ blocks), and sent for cell<br />
suspension immunophenotypic studies (flow cytometry). A portion is sent to the molecular<br />
diagnostics laboratory with an adjacent section submitted in cassette A (CMB/DNA). Additional<br />
material is sent for cytogenetic studies. The [remainder or representative sections] <strong>of</strong> the [site<br />
location] is (are) submitted in cassettes BB+ after B+ fixation and in blocks C - ______ after formalin<br />
fixation.<br />
Note: Do not use the B+ fixation if you only are submitting one block, formalin only.<br />
(NOTE: IF a frozen section was submitted by another bench, submit in cassette AFS.) Make sure you<br />
order ‘FRZ1’ in Stain Process for every frozen section block submitted<br />
Write on sides <strong>of</strong> blocks: B+ fix on B+ fixed blocks<br />
Checklist for specimens: *Make sure all containers, slides, and frozen capsules<br />
are labeled with the case # and patient’s name*<br />
__Touch preps ---2 stained (H&E) on regular<br />
slides to evaluate what type <strong>of</strong> flow panel to<br />
order<br />
__3 air dried slides on PLUS charge slides<br />
for possible Cytogenetic FISH studies<br />
___ Cassettes are ordered under ‘Block<br />
Detail’ , then select PH White (R/O<br />
Lymphoma) under engraver type<br />
__Put the Date and Time on the formalin<br />
and B+ fixative container<br />
__Snap frozen- 2 capsules without OCT<br />
compound (only if enough tissue available) ,<br />
then 1 capsule with OCT<br />
__Flow cytometry- container with RPMI<br />
(found in refrigerator) w/ copy <strong>of</strong> requistion<br />
NOTE- On call fellows/ residents: please put<br />
flow specimen on top shelf <strong>of</strong> flow fridge<br />
(located centrally in lab next to biochemical<br />
hood) and put requisition with flow decision<br />
on staining bench.<br />
_Molecular Diagnostics- container with<br />
Saline (found at grossing bench) fill out MDX<br />
form and tube to station # 370 Hours Mon-<br />
Fri 7am-345pm. After hours, put in LN fridge<br />
to be sent following day.<br />
__Cytogenetics- container with RPMI, fill out<br />
form---- tube to station #417 anytime (but<br />
after 1p Saturday-put in LN fridge).
Policy for solid tissue specimens sent to rule out lymphoma that overlaps with other Centers<br />
<strong>of</strong> Excellence<br />
In certain circumstances, specimens that fall into a non-<strong>Hematopathology</strong> Center <strong>of</strong> Excellence will either be<br />
designated “rule out lymphoma” by the surgeon or have a potential lymphoma discovered either when a frozen<br />
section is performed or when a fresh specimen is examined grossly. For example, a salivary gland may be<br />
removed and a frozen section <strong>of</strong> a mass demonstrate a dense lymphoid proliferation without evidence <strong>of</strong> a<br />
carcinoma.<br />
If an intraoperative consultation is requested, the specimen should be handled by the surgical pathology<br />
resident/fellow/pathologist on call at the hospital where the specimen has been removed.<br />
In all cases, following completion <strong>of</strong> any required intraoperative consultation, both the<br />
resident/fellow/pathologist on service for the Center <strong>of</strong> Excellence and the <strong>Hematopathology</strong> “lymph node”<br />
resident/fellow/pathologist should be notified that the specimen is in the gross room. A joint decision<br />
should be made at that time by both parties as to whom the primary pathologist should be based on factors<br />
such as the likelihood <strong>of</strong> other pathology being present or any concern that a hematopathology protocol<br />
would compromise any aspect <strong>of</strong> the pathologic examination.<br />
In cases where the Center <strong>of</strong> Excellence is not located at UPMC-Presbyterian Hospital, the decision as to<br />
whether the specimen should be handled in whole or in part by the Division <strong>of</strong> <strong>Hematopathology</strong> should be<br />
made at the originating hospital. This should either be done by members <strong>of</strong> the Center <strong>of</strong> Excellence (for<br />
example, if there is a GU specimen at UPMC-Shadyside) or after telephone agreement with the<br />
appropriate AP-related COE pathologist. This will eliminate the possibility <strong>of</strong> specimens being sent first to<br />
one hospital and then another. The resident/fellow/faculty on lymph node service should be consulted if<br />
questions occur.<br />
The <strong>Hematopathology</strong> “lymph node” resident/fellow/pathologist will be prepared to accept the responsibility for<br />
processing the entire specimen, for taking a portion <strong>of</strong> the specimen for a hematopathology workup with<br />
the remainder <strong>of</strong> the specimen grossed in by an anatomic pathology bench or functioning solely as a<br />
consultant if special procedures are deemed unnecessary (for example, if suspicion for a lymphoma is low<br />
and the tissue sample is very limited).<br />
In cases where the initial triaging to either <strong>Hematopathology</strong> or one <strong>of</strong> the AP benches seems inappropriate<br />
after sections are reviewed or ancillary studies become available, if agreeable to all parties, the primary<br />
pathologist will be switched to the pathologist on the more appropriate service. This may involve a Center<br />
<strong>of</strong> Excellence pathologist signing out a case not accessioned at their Center <strong>of</strong> Excellence, and ordering<br />
stains that need to be sent by inter-hospital courier.<br />
This model should be applied to all other Centers <strong>of</strong> Excellence where faculty, fellows or residents should act<br />
to facilitate processing.
Instructions for Fresh Tissue Biopsies Received from Outside Institutions<br />
[Revised 10/2013]<br />
Sometimes tissue specimens from outside institutions are sent to our Flow Cytometry Laboratory for cell<br />
suspension immunophenotypic studies or complete workup. In these cases, as long as sufficient tissue is<br />
available, we try to do a complete lymph node protocol on them, except that tissue is not sent to the<br />
molecular diagnostics or cytogenetics laboratory (except for cases from UPMC-Shadyside, Magee Women’s<br />
or other complete case workups that also get material sent for DNA extraction and, if appropriate, for<br />
cytogenetic studies). One must remember that if the tissue has been sent here for flow cytometric studies,<br />
sufficient tissue must be retained specifically for making a cell suspension. If the tissue submitted is very<br />
small, at a minimum, a touch imprint should be performed, and if possible, a small portion snap frozen. On<br />
cytologic specimens, if material has been retained at the referring institution, do NOT do touch preps unless<br />
there is a lot <strong>of</strong> material (several good cores) and send the entire specimen to the flow cytometry laboratory.<br />
Run the lymphoma protocol in CoPath but be sure to delete all the items that do not apply. You don’t need a<br />
CMB/DNA block unless molecular is sent. If there are any questions on a given case, please contact the<br />
pathologist covering lymph node biopsies.<br />
Cases from CHP where we are not the primary pathologists traditionally are not sectioned (CHP sections are<br />
reviewed prior to sign-out). Cases from other divisions for flow cytometry may have a section taken if there<br />
is sufficient tissue and if acceptable to the division (to better know what we are actually doing the flow<br />
cytometry on).<br />
All fresh tissue cases from UPMC-Shadyside will arrive in the Flow Cytometry lab and accessioned as UPMC-<br />
Presbyterian cases. All other cases from UPMC hospitals (and rare non-UPMC hospitals) should be<br />
accessioned as UP consults UNLESS we have the entire specimen (with or without a frozen section). If we<br />
have the entire specimen it should be accessioned as a UPMC- Presbyterian case.
Fresh Case Lymph Nodes and Other Solid Tissues Sent to UPMC-Presbyterian for Flow Cytometric<br />
Studies with a non-hematopathology Primary Pathologist [Revised 10/2013]<br />
All non-PB, non-BM, non-fluid outside cases sent to the Division <strong>of</strong> <strong>Hematopathology</strong> for flow cytometry, that<br />
are accessioned with a number that is solely being handled by the Division <strong>of</strong> <strong>Hematopathology</strong>, need not only<br />
to have the flow cytometry signed out, but a complete report in the usual format. That report should include the<br />
following components:<br />
<br />
<br />
<br />
<br />
<br />
A History which is amplified from information that is entered when the case is accessioned and should<br />
at least include the information on the requisition. It should also state: “Case received for flow<br />
cytometric consultation.”<br />
A Diagnosis - The diagnosis in cases that do not have a definitive malignant diagnosis can be "Flow<br />
cytometric immunophenotypic studies are non-diagnostic (see comment)”. Other cases may get a<br />
somewhat more definitive diagnosis, but, unless there is an actual disagreement, the diagnosis should<br />
be consistent with what the primary pathologist is calling the case. Hence, the original pathologist on<br />
almost all <strong>of</strong> these cases should be called. The diagnosis rendered can be a bit more vague than<br />
usual, for example, a follicular lymphoma may be diagnosed without giving the grade.<br />
A Comment - A comment that reiterates the diagnosis and states that the results must be correlated<br />
with the material processed at the institution <strong>of</strong> origin should be included. It may refer to the<br />
morphologic findings in the tissue processed or in the cytospin.<br />
A Microscopic Description - The description can be <strong>of</strong> the morphologic findings in the tissue that is<br />
processed or based on the cytospin, if there is nothing else. Or it can state that nothing was<br />
processed for morphological studies.<br />
A Billing code – If only H & E’s and flow are done, BC06. If additional workup or complete consultation<br />
is performed after discussion with the referring pathologist, BC03, BC04, or BC5 (See “Copath and<br />
dictation pointers” .for complete list <strong>of</strong> billing codes (BC))<br />
In addition, it must be ascertained whether or not the primary pathologist is sending more material on the case<br />
BEFORE the rest <strong>of</strong> the report is signed out. In general, some institutions do and some do not typically send<br />
additional material. This should be indicated on the requisition but it is not always clear and not always<br />
consistent.<br />
On these "BC06" cases (BC14 for the VA), permission must be obtained to order immunostains, FISH,<br />
molecular diagnostics, etc. If a full consult is requested on the requisition, IHC may be ordered. The primary<br />
pathologist should be asked about doing FISH studies and molecular diagnostics, although if the case is from a<br />
UPMC institution it should be acceptable. If a case is not from a UPMC institution, it is critical to obtain<br />
permission to order anything other than the H&Es.<br />
If you do IHC and flow was done, be sure to put in one <strong>of</strong> the Flow/IHC comments.<br />
These cases also should have a synoptic if they are lymphomas.
Helpful Hints for Handling Consult Blocks<br />
Ordering H&E slides on Consult Cases<br />
With consult cases you must order the H&E as a recut (RHHE) because the only requests that come on<br />
the Histology Special Request Log are special stains, Ipex, Hblanks, Iblanks and recuts. The slides<br />
that come with the consult case are the original H&E. We cut from the blocks that are sent, therefore it<br />
is a true recut.<br />
When consult blocks are sent for special stains/immunos enter them in Client Server as follows:<br />
-Under Stain/Process Edit/Entry:<br />
-Select the Part that corresponds to Consult Block/s NOT Consult Slide/s<br />
-Select Add Block and enter the block designation as the A, B, C – under block detail go the other<br />
block number and put outside case #.<br />
-Hint #1: An initial H&E is ordered automatically; however, it does not end up on the HISTO<br />
worklog for some reason, so will never ever get it. You must re-order it as a recut (RHHE) to<br />
actually get a slide.<br />
-Hint #2: When you Add Stain/Process to a designated block, be careful to select the correct<br />
block when ordering stains.<br />
-Sometimes you get blank slides instead <strong>of</strong> a block; check to see what Part they are accessioned and enter a<br />
designation just like a block, as above.<br />
Sending Outside Blocks to Histology<br />
All outside blocks are to be sent in yellow envelopes to Histology via the tube station # 320 with the PUH case<br />
number, patient’s name, Pathologist’s name, and <strong>Resident</strong>’s or <strong>Fellow</strong>’s name written with a sharpie pen on<br />
the envelope.
RULES FOR HISTOLOGY<br />
All exceptions must be approved by<br />
Dr. Yousem & Dr. Dhir<br />
WHEN ACCESSIONING:<br />
Pathology staff must be entered in the following order; Pathologist. (P) <strong>Fellow</strong> or Chief <strong>Resident</strong>. (R) If there is<br />
no <strong>Fellow</strong> or Chief <strong>Resident</strong> on the case enter “none”. If there is no resident, this field can be left blank.<br />
Initial H&E slides will be delivered to the listed pathologist in “reverse rank” order: 1) <strong>Resident</strong>, 2) <strong>Fellow</strong>/Chief,<br />
3) Faculty.<br />
New Bench Designations and Color Scheme; Please make sure the proper color cassette is used for<br />
appropriate handling <strong>of</strong> specimen.<br />
Hemepath – WHITE R/O Lymphoma<br />
Rush Biopsies (PUH/SYS) - PEACH<br />
Rush Neuropath (PUH) - GRAY<br />
Cytology (PUH/SYS) - GREEN<br />
ENT Routine (PUH) - PINK<br />
GI Routine (PUH) - YELLOW<br />
Muscle/Nerve Routine (PUH) - ORANGE<br />
Neuropath Routine (PUH) - BLUE<br />
Thoracic Routine (PUH) - TAN<br />
Transplant Routine (PUH) - PURPLE<br />
Autopsy (PUH) - WHITE<br />
Autopsy Neuropath (PUH) - WHITE<br />
Derm Rush (SYS) - GRAY<br />
GU (SYS) - WHITE<br />
Prostate Routine (SYS) - PINK<br />
Skins Rush (SYS) - BLUE<br />
Bone/S<strong>of</strong>t Tissue (SYS) - GREEN<br />
STAT cut <strong>of</strong>f times: The cut <strong>of</strong>f time for receiving stats in the histology lab is 11: 00AM. No exceptions, unless<br />
approved by Dr. Yousem and Dr. Dhir.<br />
When accessioning Children’s Hospital bone marrows; they must be accessioned under pediatric bone<br />
marrows, or they will be sent to the Hemepath lab, along with the other bone marrows. There will be no<br />
exceptions.
WHEN ORDERING:<br />
RE-CUTS – Must be ordered under RHHE, and the correct number in the count field. If levels are needed, this<br />
must be entered in the stain comment field when ordering the re-cuts. The cut <strong>of</strong>f time for ordering re-cuts is<br />
1:00PM to be delivered same day by the 5:30 pm courier run. Anything after 1:00PM that day will be cut and<br />
sent the following morning by the 8:30 am courier run. There will be no exceptions unless approved by Dr.<br />
Yousem & Dr. Dhir.<br />
SPECIAL STAINS – Must be ordered with the correct special stain code so there is no delay. Please<br />
remember and take into consideration that some special stains take 2 or 3 DAYS to complete. The cut <strong>of</strong>f time<br />
for ordering Histo stains is 10:00AM and will be delivered on the 2:30PM courier run that day. Anything<br />
ordered after 10:00AM will be stained the following day and delivered on the 8:30 AM courier run the following<br />
day. The only special stains performed on weekends are the Stat Grocott, AFB, and Gram. There will be no<br />
exceptions unless approved by Dr. Yousem or Dr. Dhir.<br />
IMMUNOPEROXIDASE ANTIBODIES – Must be ordered with the correct antibody code so there is no delay.<br />
Most Immunoperoxidase antibodies done in the clinical lab have the prefix A; please make sure the correct<br />
antibody is ordered. Stains ordered before 10:00AM should come out the same day. Stains ordered after<br />
10:00AM should come out by the 8:30am courier the following day.<br />
When ordering special stains, immunos, etc. on blocks that are at Shadyside, order under Shadyside and<br />
put a comment in the stain comment field saying where you want the slides sent.<br />
REPROCESSING TISSUE:<br />
Histology technicians are no longer permitted to decide if a tissue needs reprocessing. The<br />
pathologist reading the case can only make that decision. Histology must send out a suboptimal slide,<br />
which will be highlighted in green. The pathologist needs to determine if the tissue needs<br />
reprocessing. The pathologist must call Histology and give permission to reprocess. A better H&E<br />
will be sent the following morning.<br />
ORDERING BLANKS FOR INSITU:<br />
If the blanks are ordered before 11:00 AM will be sent to ISH on the 11:30AM courier run. Anything ordered<br />
after 11:00AM will be delivered to the ISH lab on the 9:30AM courier run.<br />
WHEN HISTOLOGY SENDS OUT SLIDES:<br />
All lymph node and all bone marrow slides will be sent to the Hemepath lab.<br />
All Transplant H&E slides will be sent to the case pathologist.<br />
All Neuropath H&E slides, including autopsies, will be sent to the case pathologist.<br />
All Transplant TB slides ordered will go to the case pathologist. All non-transplant TB slides will go to the<br />
resident on the case entered in the comment field.<br />
All EM kidney biopsy slides will be sent to the case pathologist.<br />
E slides will go to Cytology. All recuts, special stains, and immunoperoxidase stains will be sent to the hospital<br />
as specified in the stain comment field when ordered.<br />
All dental slides will go to the Dental department.
All recuts, special stains, and immunoperoxidase stains will be sent to the doctor ordering them. The doctors<br />
must enter in the stain comment field when ordering, the hospital where that doctor will be located to receive<br />
their slides.<br />
Comments override everything: All slides will be sent to the doctor and hospital specified in the stain comment<br />
field.<br />
For all blocks that need corrections, the appropriate department or person will be notified and they must come to the<br />
Histology lab and make the corrections. Histology will no longer be responsible for making changes to the<br />
cassettes. In addition, a specimen misidentification form must be filled out in histology.<br />
Revised 10/2013
FONT TYPE AND SIZE:<br />
All reports are typed in Arial font, size 9.<br />
GENERAL FORMATTING REQUIREMENTS<br />
GROSS DESCRIPTION<br />
The gross description is typed in sentence style, beginning at the left margin, in paragraph format.<br />
The patient’s initials are typed in all CAPS (i.e. The specimen is received in formalin labeled with the<br />
patient’s name and initials, MSP.)<br />
The label <strong>of</strong> the specimen is typed within quotation marks (i.e. The specimen is labeled “right upper<br />
lobe lung” and consists….).<br />
When multiple specimen parts are received a new paragraph is started for each part. (i.e. Part 1, Part<br />
2, etc.), using Arabic numerals.<br />
When multiple specimen parts are received each part’s gross description must include the patient's<br />
initials. (i.e. Part 1 is received labeled with the patient’s name and initials, MSP….. Part 2 is received<br />
labeled with the patient’s name and initials, MSP.)<br />
For gross specimens with a cassette listing, a period follows the last submitted description only (see<br />
example below).<br />
EXAMPLE: Gross description for a one-part specimen<br />
Gross Description<br />
The specimen is received in formalin labeled with the patient’s name, initials MSP and “rectal biopsy”. The specimen consists <strong>of</strong> an unoriented,<br />
irregular, s<strong>of</strong>t, tan, tissue fragment measuring 0.2 x 0.1 x 0.1 cm. The specimen is entirely submitted in a cassette labeled A.<br />
VGD/vat<br />
EXAMPLE: Gross description for multiple-part specimens<br />
Gross Description<br />
The specimen is received in formalin in two parts.<br />
Part 1 is labeled with the patient's name, initials MSP and “rectal biopsy”. It consists <strong>of</strong> an unoriented, irregular, s<strong>of</strong>t, tan, tissue<br />
fragment measuring 0.2 x 0.1 x 0.1 cm. The specimen is entirely submitted in a cassette labeled 1A.<br />
Part 2 is labeled with the patient's name, initials MSP and “antrum”. It consists <strong>of</strong> an unoriented, irregular, s<strong>of</strong>t, tan, tissue fragment<br />
measuring 0.2 x 0.1 x 0.1 cm. The specimen is entirely submitted in a cassette labeled 2A.<br />
VGD/vat<br />
EXAMPLE: Gross description with cassette listing<br />
Note: When a specimen part is submitted in several blocks, these are indicated by a cassette listing at the end <strong>of</strong> the gross<br />
description for that part. It is typed flush with the left margin (in the following order: part number, letter designation, a space, a dash, a<br />
space, description <strong>of</strong> tissue in block). A period follows the last submitted description only. The cassette listing is formatted as follows:<br />
The specimen is submitted as follows:<br />
1A – right upper lobe lung<br />
1B – nodule 20 cm from resection margin<br />
1C – stapled resection margin<br />
1D – lung parenchyma<br />
1E – remainder <strong>of</strong> tissue<br />
Gross Description<br />
The specimen is received in buffered formalin labeled with the patient’s name, initials MSP and “skin biopsy”. The specimen consists <strong>of</strong><br />
an un-oriented, irregular s<strong>of</strong>t tan tissue fragment measuring 0.2 x 0.1 x 0.1 cm. The following sections are submitted:<br />
1A – tips<br />
1B – ends<br />
VGD/vat<br />
EXAMPLE: Gross description for bone marrow biopsy<br />
Gross Description<br />
Received are an aspirate (part 1), biopsy, flow cytometry, cytogenetics and VNTR.<br />
Part 2, bone marrow biopsy, is received in B5 fixative, labeled with the patient's name (MSP) and site. It consists <strong>of</strong> a single core <strong>of</strong> tan,<br />
cancellous bone measuring 0.8 x 0.2 cm. The specimen is totally submitted to histology for decalcification in block A.<br />
VGD/vat
EXAMPLE: Consult material description (congross or cons) (may include a gross description)<br />
Consult Material Description<br />
Received for consultation from John Doe, M.D., are eleven (11) consult slides and nine (9) consult slides labeled S05-192; one (1)<br />
consult slide labeled S05-3303 and one (1) consult slide labeled S05-6039 from Memorial Hospital, Pathology <strong>Department</strong>, 9888<br />
Generic Avenue, Big City, PA 12345, along with an accompanying letter, surgical and cytopathology reports.<br />
VGD/vat<br />
EXAMPLE: Bone marrow biopsy clinical history (preop)<br />
Clinical History<br />
The patient is a 36-year-old male with a history <strong>of</strong> acute myelogenous leukemia, diagnosed in 04/2003 (PHB03-123456). A bone<br />
marrow performed on 02/200 (PHB05-111111) demonstrated 10% blasts. He is currently day 33 status post allogeneic peripheral<br />
blood stem cell transplant. The bone marrow is for evaluation <strong>of</strong> engraftment. Medications: Cycloporin, Diflucan, Acyclovir, Synthroid,<br />
Protonix and Magnesium.<br />
VGD/vat<br />
EXAMPLE: Consultation case, one accession number (y and preop)<br />
Clinical History<br />
The patient is a 70-year-old male.<br />
OSS S05-192, 1/7/2005, MEMORIAL HOSPITAL<br />
PRE-OP DIAGNOSIS: None given.<br />
POST-OP DIAGNOSIS: None given.<br />
PROCEDURE:<br />
Fine Needle Aspiration right neck.<br />
VGD/vat<br />
EXAMPLE: Consultation case, multiple accession numbers (y and preop)<br />
Clinical History<br />
The patient is a 70-year-old male.<br />
OSS-S05-3303, 04/14/2005, MEMORIAL HOSPITAL<br />
PRE-OP DIAGNOSIS: Left vocal cord lesion.<br />
POST-OP DIAGNOSIS: None given.<br />
PROCEDURE:<br />
Left vocal cord stripping.<br />
OSS-05-6039, 07/07/2005, MEMORIAL HOSPITAL<br />
PRE-OP DIAGNOSIS: None given.<br />
POST-OP DIAGNOSIS: None given.<br />
PROCEDURE:<br />
Left vocal cord biopsy.<br />
VGD/vat<br />
EXAMPLE: All other surgical clinical histories (preop)<br />
Clinical History<br />
Abdominal pain.<br />
PRE-OP DIAGNOSIS: Abdominal pain.<br />
POST-OP DIAGNOSIS: Same.<br />
PROCEDURE:<br />
Rectal biopsy.<br />
VGD/vat<br />
GROSS ONLY SPECIMEN REPORTS<br />
The clinical history and gross description are typed as described above in the gross description<br />
explanation.<br />
In the microscopic description field type: “No sections are submitted” or “None”.<br />
In the final diagnosis field type (in all CAPS): The specimen line, return and type the dictated final<br />
diagnosis followed by the phrase, “gross diagnosis only; see comment” within parenthesis in lower case<br />
letters (see example below).<br />
In the diagnosis comment field type the quick text code, gross and click on the running man icon to<br />
insert the standard quick text template regarding gross only specimens and further testing. Go to the<br />
pound (#) sign using the keystroke combination [ALT+F] and type in the specimen designation.<br />
INTRAOPERATIVE CONSULTATION<br />
The intraoperative consultation text field is typed in ALL CAPS (similar to the final diagnosis section).<br />
The only exceptions are: 1) the type <strong>of</strong> intraoperative consultation is typed in parentheses in<br />
lowercase letters (for example: frozen section, gross diagnosis/examination only, touch prep) and 2)<br />
the names <strong>of</strong> intraoperative staff member(s) performing the intraoperative consultation are entered
within parentheses at the end <strong>of</strong> the last line <strong>of</strong> the intraoperative consultation before the period (first<br />
initial <strong>of</strong> first name in caps and full last name with initial caps and lowercase letters - in the following<br />
order: staff pathologist/fellow/resident/PA including the title, M.D. when applicable) (see examples<br />
below).<br />
EXAMPLE: Single-part intraoperative consultation<br />
Intraoperative Consultation<br />
FS: LUNG, LEFT UPPER LOBE, LOBECTOMY (frozen section) –<br />
EXAMPLE: Intraoperative consultation with single-line diagnosis<br />
Intraoperative Consultation<br />
FS: LUNG, LEFT UPPER LOBE, LOBECTOMY (frozen section) –<br />
A. BENIGN (T. Pathologist, M.D).<br />
EXAMPLE: Intraoperative consultation with multiple diagnoses<br />
Intraoperative Consultation<br />
FS: LUNG, LEFT UPPER LOBE, LOBECTOMY (frozen section) –<br />
A. BENIGN.<br />
B. NO TUMOR SEEN (T. Pathologist, M.D. /C. <strong>Resident</strong>, M.D./A. Pa).<br />
VGD/vat<br />
EXAMPLE: Intraoperative consultation for multiple parts with multiple diagnoses<br />
Intraoperative Consultation<br />
1AFS: LUNG, LEFT UPPER LOBE, LOBECTOMY (frozen section) –<br />
A. MALIGNANT.<br />
B. SMALL CELL CARCINOMA (T. Pathologist, M.D).<br />
2ATP: LUNG, LEFT MIDDLE LOBE, LOBECTOMY (touch prep) –<br />
A. MALIGNANT (T. Pathologist, M.D.).<br />
3AGD: LUNG, LEFT MIDDLE LOBE, LOBECTOMY (gross diagnosis) –<br />
A. BENIGN (T. Pathologist, M.D.).<br />
VGD/vat<br />
EXAMPLE: Intraoperative consultation for one part with multiple frozen sections/touch preps performed on different blocks<br />
Intraoperative Consultation<br />
1AFS: SUPRAGLOTTIC MASS, LEFT, LATERAL, MEDIAL AND DEEP MARGINS (frozen section) –<br />
A. MALIGNANT.<br />
B. SQUAMOUS CELL CARCINOMA.<br />
C. TUMOR PRESENT AT LATERAL MARGIN. MEDIAL AND DEEP MARGINS FREE OF TUMOR (T. Pathologist, M.D./C.<br />
<strong>Resident</strong>, M.D.).<br />
1BFS: SUPRAGLOTTIC MASS, LEFT, INFERIOR SHAVE MARGIN (frozen section) –<br />
A. MALIGNANT.<br />
B. SQUAMOUS CELL CARCINOMA.<br />
C. TUMOR PRESENT AT THE MARGIN (T. Pathologist, M.D./C. <strong>Resident</strong>, M.D.).<br />
1CFS: SUPRAGLOTTIC MASS, ANTERIOR SHAVE MARGIN (frozen section) –<br />
A. MALIGNANT.<br />
B. SQUAMOUS CELL CARCINOMA.<br />
C. TUMOR EXTENDS VERY CLOSE IF NOT TO THE CAUTERIZED MARGIN (T. Pathologist, M.D./C. <strong>Resident</strong>, M.D.).<br />
VGD/vat<br />
FINAL DIAGNOSIS<br />
The Final Diagnosis is typed in ALL CAPS and bold type. The exception: Any text placed within<br />
parentheses that is non-diagnostic {i.e. (see comment)} is in lowercase bold type.<br />
The specimen line identifies the specimen, identifies the location <strong>of</strong> the specimen, and indicates the<br />
procedure performed to remove the specimen and is aligned with the left margin. Following the type <strong>of</strong><br />
excision, type a space, a dash and then return.<br />
Each diagnosis ends in a period.<br />
A single diagnosis is aligned flush with the left margin and not indented.<br />
For multiple diagnoses, each diagnosis is given a letter designation (A, B, C, etc.).
For multiple parts with one or more diagnoses, each part is labeled as PART # and followed by a colon<br />
mark and a single space and the specimen line (i.e. PART 1: LUNG…). Each diagnosis is given a<br />
letter designation (A, B, C, etc.), with the exception <strong>of</strong> a diagnosis with only one line. The letter<br />
designation is aligned with the beginning <strong>of</strong> the specimen header in a hanging indent, (created using<br />
the keystroke combination [CTRL+M]), followed by a period and a space, and then the diagnosis.<br />
Note: When the hanging indent is created, hitting return at the end <strong>of</strong> the line A diagnosis will create<br />
the appropriate indent for the remaining diagnosis lines.<br />
A blank line is inserted between parts.<br />
For consult diagnoses, place the outside slide number and the accession date <strong>of</strong> the outside slide<br />
number in parenthesis next to the diagnosis specimen line.<br />
Expand abbreviations in the final diagnosis field. Example: If the dictator says CIN 1 type CERVICAL<br />
INTRAEPITHELIAL NEOPLASIA 1 (CIN 1).<br />
EXAMPLE: Final diagnosis heading - specimen part type, location <strong>of</strong> specimen, procedure performed<br />
Final Diagnosis<br />
LUNG, LEFT UPPER LOBE, LOBECTOMY –<br />
EXAMPLE: Final diagnosis for one part with one diagnostic line<br />
Final Diagnosis<br />
LUNG, LEFT UPPER LOBE, LOBECTOMY –<br />
BENIGN.<br />
VGD/vat<br />
EXAMPLE: One part, multiple diagnosis lines<br />
Final Diagnosis<br />
SOFT TISSUE KNEE MASS, RIGHT, EXCISION –<br />
A. SKIN AND SUBCUTANEOUS TISSUE WITH FIBROUS-WALLED CYST WITH FOCAL RUPTURE,<br />
B. SYNOVIAL CELL PROLIFERATION AND RARE NON-VIABLE BONY SPICULES OF GANGLION/SYNOVIAL CYST, 11.5 X<br />
3.8 CM (see comment).<br />
C. NEGATIVE FOR MALIGNANCY.<br />
VGD/vat<br />
EXAMPLE: Multiple parts, one or more diagnosis lines<br />
Final Diagnosis<br />
PART 1: GALLBLADDER, CHOLECYSTECTOMY –<br />
CHOLELITHIASIS AND MILD CHRONIC CHOLECYSTITIS.<br />
PART 2: LIVER, RANDOM NEEDLE BIOPSY –<br />
A. NON-CIRRHOTIC LIVER TISSUE WITH MICROVESICULAR AND MACROVESICULAR STEATOSIS INVOLVING 30%<br />
OF THE HEPATOCYTES.<br />
B. MILD NONSPECIFIC PORTAL CHRONIC INFLAMMATION (see microscopic description).<br />
VGD/vat<br />
EXAMPLE: Bone marrow biopsy final<br />
Final Diagnosis<br />
PART 1: PERIPHERAL BLOOD –<br />
WITHIN NORMAL LIMITS.<br />
PARTS 2<br />
AND 3: BONE MARROW, BIOPSY AND ASPIRATE –<br />
TRILINEAGE HEMATOPOIESIS WITH FOCI OF LYMPHOCYTOSIS INCLUDING LYMPHOID AGGREGATES (see<br />
comment).
VGD/vat<br />
EXAMPLE: Consultation diagnosis, one part, one accession number<br />
Final Diagnosis<br />
RIGHT AND LEFT OVARY, BILATERAL SALPINGO-OOPHORECTOMY (OSS S05-6612, 06/14/05) –<br />
A. POORLY-DIFFERENTIATED INTRACYSTIC CARCINOMA WITH FEATURES OF SEROUS AND ENDOMETRIOID<br />
CARCINOMA.<br />
B. ADENOCARCINOMA APPEARS TO BE INTRACYSTIC WITHOUT EVIDENCE OF OVARIAN SURFACE INVOLVEMENT ON<br />
PROVIDED SLIDES.<br />
C. LYMPHOVASCULAR INVASION IS NOT IDENTIFIED.<br />
D. LEFT FALLOPIAN TUBE, HISTOLOGICALLY UNREMARKABLE.<br />
E. RIGHT OVARY AND RIGHT FALLOPIAN TUBE WITH PHYSIOLOGIC CHANGES.<br />
F. DEFINITE ENDOMETRIOSIS IS NOT IDENTIFIED.<br />
VGD/vat<br />
EXAMPLE: Consultation diagnosis, multiple parts one accession number<br />
Final Diagnosis<br />
PART 1: ENDOCERVIX, CURETTINGS (OSS S05-1191, 07/07/05) –<br />
ECTOCERVICAL AND ENDOCERVICAL TISSUE FRAGMENTS WITH NO SIGNIFICANT PATHOLOGIC ABNORMALITY.<br />
PART 2: ENDOMETRIAL CURETTINGS (OSS S05-1191, 07/07/05) –<br />
ATYPICAL COMPLEX HYPERPLASIA IN A BACKGROUND OF SECRETORY PATTERN ENDOMETRIUM WITH TUBAL<br />
AND CLEAR CELL METAPLASIA.<br />
VAT/vat<br />
EXAMPLE: Consultation diagnosis, multiple parts, multiple accession numbers<br />
Final Diagnosis<br />
PART 1: FINE NEEDLE ASPIRATION OF RIGHT NECK (OSS S05-192, 01/07/05) –<br />
A. SATISFACTORY FOR INTERPRETATION.<br />
B. NEGATIVE FOR MALIGNANT CELLS.<br />
C. FRAGMENTS OF FIBROADIPOSE TISSUE.<br />
PART 2: VOCAL CORD, LEFT, STRIPPING (OSS S05-3303, 04/14/05) –<br />
KERATOSIS WITH MILD DYSPLASIA.<br />
PART 3: VOCAL CORD, LEFT, BIOPSY (OSS S05-6039, 07/07/05) –<br />
HYPERPLASTIC, CHRONICALLY INFLAMED SQUAMOUS MUCOSA.<br />
VGD/vat<br />
DIAGNOSIS COMMENT<br />
The diagnosis comment field is typed in sentence style in bold type, aligned with the left margin, in<br />
paragraph format and may contain quick text or free text transcription.<br />
EXAMPLE: Diagnostic comment<br />
Diagnosis Comment<br />
The histologic features seen in this material are consistent with quiescent ulcerative colitis. No dysplasia is seen.<br />
VGD/vat
CLINICAL HISTORY<br />
<br />
<br />
<br />
<br />
<br />
In the clinical history field, standard quick text templates are used. Type the quick text preop for bone<br />
marrows, consultations and all other surgical cases) and click on the running man icon to insert the<br />
standard quick text template for the patient's clinical history. Go to the pound (#) signs using the<br />
keystroke combination [ALT+F] and type in the dictated information.<br />
The clinical history is typed sentence style (initial uppercase, remainder lowercase; see examples<br />
below).<br />
The patient’s age is typed with hyphens (i.e. The patient is a 24-year-old female).<br />
The first word in the pre-op, post-op and procedure are capitalized and each line ends with a period<br />
(see examples below).<br />
Consultation cases include the outside slide number (OSS), outside slide accession date and the name<br />
<strong>of</strong> the hospital requesting the consult between the clinical history line and the preoperative diagnosis<br />
line in all capital letters and in bold typeface.<br />
SYNOPTICS<br />
A synoptic should be added to all bone marrow and solid tissue reports when a<br />
hematopathologic/lymphoid neoplasm is being diagnosed. Currently on the bone marrows, these are<br />
being added on first-time diagnoses only, although they do not need to be limited to those cases.<br />
Instructions for the use <strong>of</strong> synoptics are available online. The CoPath user guide is posted on the<br />
CoPathPlus website http://copath.upmc.com under the link for CoPathPlus Training Resources. The<br />
Synoptic Data Entry and Reporting Chapter is Chapter 24.<br />
A hard copy is available in rooms G315 and G323.<br />
There are currently four (4) synoptics used in <strong>Hematopathology</strong>.<br />
o Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders Synoptic<br />
o Gastrointestinal Lymphoma Resection Synoptic<br />
o Hodgkin Lymphoma Biopsy/Staging Synoptic<br />
o Non-Hodgkin’s Lymphoma Biopsy/Resection Synoptic<br />
OTHER<br />
Never use tabs.<br />
Do not change FONT or center justify header in IHC table.
LYMPH NODE/SOLID TISSUE ORGANIZATION OF SIGNOUT MATERIAL<br />
(SLIDES, BLOCKS, PAPERWORK)<br />
1.0 Principle<br />
1.1 All material, whether in-house or consult, received fresh or fixed (glass slides and/or blocks)<br />
must be kept in an organized fashion with detailed records to facilitate efficient and accurate<br />
functioning <strong>of</strong> lymph node/solid tissue hematopathology sign out service.<br />
2.0 Specimen Requirements<br />
2.1 Specimens include all cases designated for hematopathology lymph node/solid<br />
tissue service.<br />
3.0 Reagents, Supplies and Equipment<br />
3.1 Reagents<br />
Not Applicable<br />
3.2 Supplies<br />
<strong>Hematopathology</strong> Case Worksheets<br />
3.3 Equipment<br />
Computer with Micros<strong>of</strong>t Office<br />
4.0 Calibration and Standardization<br />
Not applicable<br />
5.0 Quality Control<br />
5.1 All problems noted in terms <strong>of</strong> special handling, transcription or histology will be entered under<br />
the “Adverse Event” section for each case in CoPath.<br />
(See Appendix G - Adverse Event Recording)<br />
6.0 Procedure<br />
6.1 Throughout the day, all material for the lymph node service is brought into the lymph node sign<br />
out room (G323) to be distributed with the cases.<br />
6.1.1 If formalin fixed H & E stained slides from tissue grossed and submitted the day before<br />
are not received by 4:00PM, follow up with a call to histology laboratory to determine<br />
when the slides will be available (extension 647-7660).<br />
6.2 Whenever slides are received, place on a tray with all other slides on the case together with all<br />
paperwork and place on the microscope table.<br />
6.2.1 If a <strong>Hematopathology</strong> Case Worksheet (Appendix B) already exists because the case<br />
was handled in the gross room or the sheet was filled out when the consult was<br />
accessioned/received, record which slides were received.<br />
6.2.2 If a case does not already have a <strong>Hematopathology</strong> Case Worksheet, print one out<br />
in Copath under the “Heme Worksheet” function.
6.2.3 Determine if any slides that should be present are missing by one <strong>of</strong> the following<br />
methods:<br />
6.2.3.1 Check gross description and current time. Rush formalin fixed and B+ fixed H&E<br />
stains come out by the next day after submission. PAS should be out during the<br />
afternoon on the same day.<br />
6.2.3.2 Check the <strong>Hematopathology</strong> Case Worksheet for date/time stains were ordered.<br />
Assuming the block is in histology, immunostains ordered before 9AM should be out late<br />
on the same day (does not always happen) and those ordered by 6PM should be out<br />
early the following day. In situ hybridization stains are typically expected in 2 days.<br />
6.2.3.3 Print out the "CoPath Accession Log with stain information by specimen"<br />
(Appendix H) after ordering the stains for the case. Check pending stains noting if all<br />
verified stains have been received. (If not, call histology/immunohistology laboratory ext<br />
7-7663) and if all unverified stains are not overdue (See 6.3.3.1 and 6.3.3.2 for expected<br />
turn-around times and follow-up instructions).<br />
6.2.3.3.1 Directions for CoPath called: “Accession Log with Information by Specimen”<br />
1. Log onto CoPath.<br />
2. Click on FILE in the menu bar.<br />
3. Choose BROWSE ITEMS.<br />
4. Under the Browse Items list, find the report "Accession Log w/Stain Info by<br />
Specimen"<br />
5. Click and drag the “Accession Log w/Stain Info by Specimen” report into your<br />
menu on the left.<br />
6. Double click on it to open the report.<br />
7. The next time you log on, you will be able to directly access the report from<br />
your menu list.<br />
6.2.3.3.2 Directions for bringing up specific "Accession Log w/Stain Information" report:<br />
1. Under Data Field, highlight "Specimen."<br />
2. Choose Individual Items as selection method and type the specimen number<br />
in the "Select a Specimen" Box.<br />
3. Click OK.<br />
4. Under Data Field, for "Retrieval Flag","Stain/Process" and "Request Class",<br />
choose All Values as Selection Method.<br />
5. Click OK and report will appear.<br />
6.2.3.4 Attach copy <strong>of</strong> print out to <strong>Hematopathology</strong> Case Worksheet, with notation <strong>of</strong><br />
any follow-up performed.<br />
All cases (slides, paperwork, folder) will be kept in one <strong>of</strong> the following shelves:<br />
Pending flow (inhouse or consult fresh tissue)<br />
Pending IHC cases (keep separated whether out today or the next day)<br />
Pending receipt <strong>of</strong> requested outside blocks/blanks/slides (Go through at least<br />
every other day.)<br />
Pending H&E’s ONLY<br />
Pending Molecular and Cytogenetic studies<br />
Dictated, to Pro<strong>of</strong>-read<br />
Signed out cases, pending Immunostains/ISH/ MDX/ Cytogenetics for addendums
PHS13-<br />
Blocks in Histology<br />
<strong>Hematopathology</strong> Case Worksheet<br />
PATIENT:<br />
Blocks sent down to histology<br />
Date/Time<br />
Blanks sent down to histology<br />
Date/Time<br />
Requested Date:<br />
Blocks<br />
Blanks<br />
Other<br />
Contact:<br />
ROUTINE STAINS/BLANKS<br />
Stain Code Block(s) Requested Ordered Received Comments<br />
H & E<br />
RHHE<br />
PAS<br />
HPAS<br />
Grocott<br />
HGRO<br />
AFB<br />
HAFT<br />
Blanks (# ) HBNKNC<br />
Congo Red<br />
HCNR<br />
Steiner<br />
HSTNC<br />
IMMUNOSTAINS * = run stain process group<br />
Stain Code Block(s) Requested Ordered Received Comments<br />
Small B-Cell Panel<br />
CD3, 20,5,10,43<br />
BCL-2,BCL-6,cycD1<br />
SBCP*<br />
MALT Eval<br />
anti-kappa, anti-lambda<br />
CD20, BCL-2, CD43, CD5<br />
BCL-6, CD10, cyclin D1, CD3<br />
DLBCL Eval<br />
CD20, BCL-6, MUM-1<br />
CD3, BCL-2, CD10<br />
Cyclin D1, CD5, MYC, Ki-67<br />
Cytoplasmic Ig-IHC<br />
anti-kappa, anti-lambda<br />
Cytoplasmic Ig-ISH<br />
Kappa, Lambda, MRNA<br />
Myeloma<br />
Kappa BM, Lambda BM<br />
CD138<br />
AMALTe*<br />
ADLBCL*<br />
ACIG*<br />
ICIG*<br />
AMyel*<br />
Double k/lambda IKPLM *<br />
Ig Heavy Chains<br />
AIGH*<br />
anti-IgG,IgA, IgM, IgD<br />
Anti-IgA<br />
Anti-IgD<br />
Anti-IgG<br />
Anti-IgG4<br />
Anti-IgM<br />
CD20/L26<br />
CD22<br />
CD79a<br />
PAX 5<br />
CD21<br />
CD23<br />
CD138<br />
AIGA<br />
AIGD<br />
AIGG<br />
AIGG4<br />
AIGM<br />
AL26<br />
ACD22<br />
ACD79<br />
APAX5<br />
ACD21<br />
ACD23<br />
CD138<br />
UPMC Page 1 <strong>of</strong> 4
J chain<br />
AJ<br />
BCL2<br />
ABCL2<br />
BCL2 E17<br />
ABCL2E17<br />
MYC<br />
c-MYC<br />
BCL6<br />
ABCL6<br />
CD10<br />
ACD10<br />
IRF4/MUM-1<br />
AMUM1<br />
Cyclin D1<br />
ACYCLD1<br />
SOX-11<br />
SOX11<br />
P27<br />
AP27<br />
LEF-1/TCF-1<br />
LEF-1<br />
Annexin A1<br />
AANNEX<br />
Mini-ALL<br />
AAllm*<br />
TdT, CD34<br />
TdT<br />
ATDT<br />
CD45/LCA<br />
ALCA<br />
Hodgkin's Panel<br />
AHODPP*<br />
CD3, 20, 15, 30<br />
EMA, LCA<br />
HL expanded<br />
AHL*<br />
CD20, CD3, CD45/LCA,<br />
CD30/Ber H2, CD15/Leu M1,<br />
PAX 5, MUM-1,OCT-2, Bob.1, EMA<br />
OCT-2/Bob.1<br />
AOCT*<br />
HL, extras<br />
AHLe*<br />
PAX5, MUM-1, OCT-2<br />
Bob.1<br />
CD15/Leu M1<br />
ALEUM1<br />
CD30/Ber H2<br />
ACD30<br />
EMA<br />
AEMA<br />
ALCL<br />
AALCL*<br />
ALK-1, Clusterin<br />
Alk-1<br />
Clusterin<br />
Pan T-Cell/Basic subset<br />
<strong>Hematopathology</strong> Case Worksheet<br />
AALK1<br />
ACLUST<br />
APANT*<br />
CD2, CD3, CD4, CD5<br />
CD7, CD8<br />
T-cell subsets other<br />
ATCSS*<br />
Beta-F1, CD57, CD25, Granzyme B,<br />
CD56, TIA1, PD-1, CXCL 13<br />
NK lymphoma<br />
CD20/L26, CD2, CD3, CD5<br />
CD7, Beta-F1, CD56, TIA1<br />
Granzyme B<br />
EBV-ISH (EBER)<br />
CD1a<br />
CD2<br />
CD3<br />
CD4<br />
CD5<br />
CD7<br />
CD8<br />
ANKL*<br />
ACD1a<br />
ACD2<br />
ACD3<br />
ACD4<br />
ACD5<br />
ACD7<br />
ACD8<br />
UPMC Page 2 <strong>of</strong> 4
CD43/Leu 22<br />
CD279/PD-1<br />
CXCL13<br />
Beta-F1<br />
Gamma TCR<br />
CD56<br />
CD57/Leu 7<br />
TIA1<br />
Granzyme B.<br />
CD25<br />
Myeloblast eval<br />
MPO, Lyso, Neut elast<br />
CD117, CD34, TdT<br />
Myeloblast-limited<br />
MPO, CD117, CD34, TdT<br />
Mini-Myeloblasts<br />
CD117, CD34<br />
BM Lineage<br />
CD68/PGM-1, MPO, CD34<br />
Glycophorin A,Factor VIIIRA<br />
ACD43<br />
PD1<br />
ACXCL13<br />
ABF1<br />
ACD56<br />
ALEU7<br />
ATIA<br />
AGRANZ<br />
ACD25<br />
AMyBe*<br />
AMyBL*<br />
AMyBm*<br />
ABML*<br />
CD68/PGM-1<br />
ACD68<br />
CD123<br />
CD123<br />
TCL-1<br />
TCL-1A<br />
Myeloperoxidase<br />
AMPO<br />
Lysozyme<br />
ALYSO<br />
CD14<br />
CD14<br />
CD163<br />
CD163<br />
CD33<br />
ACD33<br />
Neut. Elast<br />
ANE<br />
CD117<br />
ACKIT<br />
Tryptase<br />
ATRYP<br />
CD34<br />
ACD34<br />
Glycophorin A<br />
AGLYP<br />
Factor VIIIRA<br />
AVWF<br />
CD61<br />
ACD61B<br />
Ki-67/MIB-1<br />
AKi67<br />
P53<br />
AP53<br />
AE1/AE3<br />
AAE13<br />
Cam 5.2<br />
ACAM<br />
Pankeratin<br />
APANKR<br />
S100/S100a<br />
AS100D<br />
CD207 / Langerin<br />
LANG<br />
EBV-ISH (EBER) IEBER *<br />
EBV-LMP<br />
AEBV<br />
Parvovirus<br />
APARVO<br />
CMV<br />
ACMV<br />
HHV8<br />
AHHV8<br />
H.Pylori<br />
HPYL<br />
Bartonella (CSD)<br />
BHENS<br />
Treponema<br />
ATREP<br />
Herpes Simplex 1/2<br />
AHSV12<br />
UPMC
MOLECULAR<br />
DNA/RNA (Already Stored) Frozen Blank Slides Available Order blanks - See PageOne<br />
TEST<br />
Ig heavy chain and light chain gene rearrangment, PCR<br />
T-cell receptor gamma chain rearrangment, PCR<br />
T-cell receptor beta chain gene rearrangment, PCR<br />
JAK2 V617F<br />
BCR-ABL1<br />
PML-RARA<br />
FLT3 & NPM1<br />
CLL sequencing for somatic hypermutation<br />
Req'd Ord'dTissue/Slides Sent(date/time) Comments<br />
CYTOGENETICS Blank Slides Available Order Blanks - See Page One<br />
Break Apart Rearrangement Probes<br />
Other<br />
ALK (2p23) RARA (17q21) ASS deletion (9q34)<br />
AML1 (21q22) TCRB (7q34) ATM deletion (11q22.3)<br />
BCL2 (18q21) TCRG (7p14) CEP X/Y<br />
BCL3 (19q13) TCRAD (14q11) D7S486/CEP7 -7/7q31 I (7q)<br />
BCL6 (3q27) TCR3 (19p13) (D7S522/CEP7) - 7/7q31<br />
BCL10 (1p22) TCL1(14q32.1) Deletion 13q (13q14) D135319<br />
CBFB (16q22)<br />
Deletion 20q (20q12) D205108<br />
CCND1 (11q13) Translocation Probes EGR1 -5/5q-<br />
ETV6 (TEL) (12p13) RUNX1T1/RUNX1 (ETO/AML1) t(8;21) AML FIP1L1-CHIC2-PDGFRA(4q12) (CHIC2 deletion)<br />
EVI1 (MECOM) (3q26.2) BIRC3-MALT1 (API2-MALT1) t(11;18) CDKN2A (p16) (9p21 deletion)<br />
EWSR1 (22q11.2) BCR-ABL t(9;22) CML/ALL/AML TP53 deletion (17p13.1)<br />
FGFR1 (8p12) IGH - CCND1 (T11:14) Trisomy 5, 9, 15<br />
IGH (14q32) IGH-BCL2 t(14;18) Trisomy 8 D8Z2<br />
IGK (2p11) CBFB-MYH1 (16p13/16q22) t(16;16). inv(16) Trisomy 12 D1273<br />
IGL (22q11)<br />
IGH-FGFR3 t(4;14)<br />
MALT1 (18q21)<br />
IGH-MAF t(14;16)<br />
MLL (11q23)<br />
IGH MALT1 t(14;18)<br />
MYC (8q24)<br />
IGH-MYC t(8;14)<br />
MYCN (2P23-24) (for amp)<br />
PML-RARA t(15;17)<br />
PAX5 (9p13)<br />
TEL-AML1 (ETV6/RUNX1) t(12;21)<br />
PDGFRB (5q33)<br />
Panels<br />
B-Cell Lymphoma - BCL6 (3q27) / MYC (8q24) / IGH (14q32.3) / BCL2 (18q21)<br />
CLL - MYB (6q23) / ATM (11q22.3) / trisomy 12 / D13S319 (13q14.3) / IGH (14q32.3) / p53 (17p13.1)<br />
Multiple Myeloma MM - D13S319 (13q14.3) / ATM (11q22.3) / p53 (17p13.1) / IGH (14q32.3) / CCND1 (11q13)<br />
Childrens' ALL - CEP4/CEP10/CEP17 (trisomies 4, 10, 17) / BCR/ABL [t(9;22)] / MLL (11q23) / TEL-AML1 (ETV6/RUNX1) t((12;21)<br />
Childrens' AML - EGR1 (5q31) / monosomy 7 / ETO-AML1 (RUNX1T1/RUNX1) [t(8;21)] / MLL (11q23) / CBFB [inv(16)]<br />
MDS/AML - EGR1 (5q31) / D7S486 (7q31)-CEP7 / trisomy 8 / D2OS108 (20q12)<br />
AML Panel - EGR1 (5q31) / D7Z1 - D7S486 / RUNX1T1-RUNX1[t(8;21)] / CBFB [inv(16) or t(16;16)] / MLL [t(11;var)]<br />
[as needed (i.e. if not seen by classical analysis)]<br />
MPD panel - BCR-ABL1 / CEP8 / CEP9 / D2OS108 (20q12)<br />
Myeloid/lymphoid neoplasm with eosinophilia - FIP1L1-CHIC2-PDGFRA (4q12), PDGFRB (5q33), FGFR1 (8p13)<br />
Additional FISH Probes Request (please list) Requested Ordered Blanks Sent<br />
Comments
Filing <strong>of</strong> Slides<br />
All slides from cases signed out by the <strong>Hematopathology</strong> Division must be placed for filing in the bone<br />
marrow laboratory (PUH G325.1).<br />
Location <strong>of</strong> Lymph Node slides<br />
Before 1992 (LN were part <strong>of</strong> Surgical Pathology Section) - Iron Mountain<br />
SEPT 1992 to PHS11-34754<br />
- Iron Mountain<br />
PHS11- 34813 - present<br />
-G325.1 Bone marrow<br />
Reading room PUH<br />
Starting April, 2009 - LN surgical pathology requisitions are stored in the CLB, the file drawer<br />
behind the grossing station.
Lymph Node Rotation Checklist<br />
Note: Items indicated in BOLD constitute the basic knowledge expected <strong>of</strong> residents rotating in<br />
<strong>Hematopathology</strong>. Additional (non-bolded) items should also be included for advanced learners<br />
including fellows.<br />
Orientation with Dr. Swerdlow (Division Director) or his designate.<br />
Done prior rotation<br />
Orientation by experienced trainee.<br />
Done prior rotation<br />
Orientation by Lymph Node Assistant or designate.<br />
Done prior rotation<br />
Competent and comfortable with gross processing and triaging <strong>of</strong> fresh lymph nodes and<br />
spleens and other specimens with possible hematopoietic/lymphoid disorders.<br />
Competent and comfortable to construct, dictate and pro<strong>of</strong> full reports and to order stains using<br />
CoPath.<br />
Knows normal architecture and immunoarchitecture <strong>of</strong> lymph node, spleen and Peyer’s Patch.<br />
Knows reactivity <strong>of</strong> all antibodies used in flow cytometry panel to assess hematopoietic/lymphoid<br />
disorders in lymph node, spleen and all other extranodal sites (panels in resident/fellow<br />
handbook).<br />
Confidently can interpret flow cytometry data including histograms.<br />
Knows role <strong>of</strong> cytogenetic and molecular diagnostic studies in evaluating<br />
hematopoietic/lymphoid disorder in lymph node, spleen and other extranodal sites.<br />
Learned diagnostic criteria using multiparameter approach and clinical implications for<br />
each <strong>of</strong> the following in lymph nodes and extranodal sites (residents to<br />
accomplish at least lower case items in bold, fellows to accomplish all over one<br />
year):<br />
Diagnosis / Finding<br />
Observed<br />
Actual<br />
Case<br />
Observed<br />
Teaching<br />
File Case<br />
Read<br />
about<br />
NON-NEOPLASTIC DISORDERS (NODAL)<br />
Viral-associated adenitis (infectious mononucleosis,<br />
postvaccinal, herpes zoster, cytomegalovirus,<br />
<br />
measles, HIV)<br />
Syphilis <br />
Toxoplasmosis<br />
<br />
Cat-scratch disease
Diagnosis / Finding<br />
Observed<br />
Actual<br />
Case<br />
Observed<br />
Teaching<br />
File Case<br />
Read<br />
about<br />
Granulomatous processes <br />
Whipple’s disease <br />
Bacillary angiomatosis <br />
Autoimmune disorders (Rheumatoid arthritis,<br />
Sjögren’s syndrome, Adults Still’s disease, systemic<br />
lupus erythematosus)<br />
Sarcoidosis<br />
<br />
<br />
Angi<strong>of</strong>ollicular hyperplasia/Castleman’s Disease <br />
Inflammatory pseudotumor <br />
Dermatopathic lymphadenopathy <br />
Sinus histiocytosis with massive adenopathy<br />
(Rosai-Dorfman)<br />
<br />
Histiocytic Necrotizing Lymphadenitis <br />
Kimura’s disease/angiolymphoid hyperplasia with<br />
eosinophilia<br />
<br />
Drug reactions (Dilantin, Tegretol) <br />
Hemophagocytic syndrome <br />
Immunodeficiency states (including ALPS, other) <br />
Lymph node infarction <br />
MALIGNANT LYMPHOMAS<br />
B lymphoblastic leukaemia/lymphoma <br />
T lymphoblastic leukaemia/lymphoma <br />
Chronic lymphocytic leukaemia/small<br />
lymphocytic lymphoma<br />
Lymphoplasmacytic lymphoma<br />
Extranodal marginal zone lymphoma <strong>of</strong> mucosaassociated<br />
lymphoid tissue (MALT lymphoma)<br />
<br />
<br />
<br />
Nodal marginal zone lymphoma <br />
Follicular lymphoma
Diagnosis / Finding<br />
Observed<br />
Actual<br />
Case<br />
Observed<br />
Teaching<br />
File Case<br />
Read<br />
about<br />
Primary cutaneous follicle centre lymphoma <br />
Mantle cell lymphoma <br />
Diffuse large B-cell lymphoma (DLBCL), NOS <br />
T-cell/histiocyte rich large B-cell lymphoma <br />
Primary DLBCL <strong>of</strong> the CNS <br />
Primary cutaneous DLBCL, leg type <br />
EBV positive DLBCL <strong>of</strong> the elderly <br />
DLBCL associated with chronic inflammation <br />
Lymphomatoid granulomatosis <br />
Primary mediastinal (thymic) large B-cell<br />
lymphoma<br />
<br />
Intravascular large B-cell lymphoma<br />
<br />
ALK positive DLBCL <br />
Plasmablastic lymphoma <br />
Large B-cell lymphoma arising in associated<br />
multicentric Castleman disease<br />
<br />
Primary effusion lymphoma <br />
Burkitt lymphoma <br />
B-cell lymphoma, unclassifiable, with features<br />
intermediate between diffuse large B-cell lymphoma<br />
and Burkitt lymphoma<br />
<br />
B-cell lymphoma, unclassifiable, with features<br />
intermediate between diffuse large B-cell lymphoma<br />
and classical Hodgkin lymphoma<br />
<br />
Adult T-cell leukaemia/lymphoma <br />
Extranodal NK/T cell lymphoma, nasal type <br />
Enteropathy-associated T-cell lymphoma <br />
Hepatosplenic T-cell lymphoma <br />
Subcutaneous panniculitis-like T-cell lymphoma
Diagnosis / Finding<br />
Observed<br />
Actual<br />
Case<br />
Observed<br />
Teaching<br />
File Case<br />
Read<br />
about<br />
Mycosis fungoides<br />
<br />
Sézary syndrome <br />
Primary cutaneous CD30 positive T-cell<br />
lymphoproliferative disorders<br />
<br />
Lymphomatoid papulosis <br />
Primary cutaneous anaplastic large lymphoma <br />
Primary cutaneous gamma-delta T-cell lymphoma <br />
Primary cutaneous CD8 positive aggressive<br />
epidermotropic<br />
<br />
Primary cutaneous CD4 positive small/medium T-cell<br />
lymphoma<br />
<br />
Peripheral T-cell lymphoma, NOS <br />
Angioimmunoblastic T-cell lymphoma <br />
Anaplastic large cell lymphoma, ALK positive <br />
Anaplastic large cell lymphoma, ALK negative <br />
Nodular lymphocyte predominant Hodgkin<br />
lymphoma<br />
<br />
Classical Hodgkin lymphoma <br />
POST-TRANSPLANT LYMPHOPROLIFERATIVE<br />
Early lesions <br />
Plasmacytic hyperplasia <br />
Infectious-mononucleosis-like PTLD <br />
Polymorphic PTLD<br />
<br />
Monomorphic PTLD, B-cell types, T-cell types<br />
<br />
Hodgkin lymphoma type PTLD <br />
HISTIOCYTIC AND DENDRITIC CELL<br />
Histiocytic sarcoma <br />
Langerhans cell histiocytosis/sarcoma
Diagnosis / Finding<br />
Observed<br />
Actual<br />
Case<br />
Observed<br />
Teaching<br />
File Case<br />
Read<br />
about<br />
Interdigitating dendritic cell sarcoma <br />
Follicular dendritic cell sarcoma <br />
OTHER NEOPLASMS<br />
Mastocytosis <br />
Myeloid sarcoma <br />
Blastic Plasmacytoid dendritic cell tumor <br />
Metastatic tumors <br />
Learned diagnostic criteria for the following in the SPLEEN (residents to accomplish at least<br />
items in lower case bold, fellows to accomplish all over one year):<br />
Diagnosis / Finding<br />
BENIGN<br />
Localized lymphoid hyperplasia<br />
Changes in benign systemic and infectious disorders<br />
Observed<br />
Actual<br />
Case<br />
Observed<br />
Teaching<br />
File Case<br />
Read<br />
about<br />
<br />
<br />
Rheumatoid arthritis (Felty’s syndrome) <br />
Autoimmune thrombocytopenic purpura<br />
<br />
Thrombotic thrombocytopenic purpura <br />
Hereditary spherocytosis <br />
Acquired immune deficiency syndrome <br />
Bacterial infections including bacillary angiomatosis <br />
Viral infections including infectious<br />
mononucleosis<br />
<br />
Castleman disease <br />
Fibrocongestive splenomegaly <br />
Histiocytic proliferations <br />
Lipid histiocytes <br />
Ceroid histiocytosis
Gaucher’s disease<br />
<br />
Langerhans cell histiocytosis <br />
Hemophagocytic syndrome <br />
SPLENIC EXPRESSION OF THE FOLLOWING<br />
LYMPHOMAS<br />
Chronic lymphocytic leukemia / Small<br />
lymphocytic lymphoma<br />
<br />
Lymphoplasmacytic lymphoma <br />
Mantle cell lymphoma<br />
Splenic and other marginal zone B-cell<br />
lymphomas<br />
<br />
<br />
Splenic lymphoma/leukaemia, unclassifiable <br />
Follicular lymphoma<br />
<br />
Diffuse large B-cell lymphoma <br />
Hepatosplenic T-cell lymphoma <br />
Other non-Hodgkin’s lymphomas <br />
Prolymphocytic leukemia <br />
Large granular lymphocytic leukemia <br />
CHANGES IN LEUKEMIAS AND<br />
MYELOPROLIFERATIVE DISORDERS<br />
Chronic myeloid leukemia <br />
Chronic myeloproliferative neoplasm <br />
Hairy cell leukemia <br />
Acute leukemias <br />
Systemic mastocytosis<br />
NONHEMATOPOIETIC LESIONS<br />
Developmental cysts<br />
Developmental hamartomas<br />
Vascular neoplasms<br />
<br />
<br />
<br />
Hemangiomas<br />
<br />
Lymphangiomas <br />
Littoral-cell angiomas
Angiosarcomas <br />
Nonvascular sarcomas <br />
Metastases <br />
Inflammatory pseudotumor
PRESENTED THE FOLLOWING “LYMPH NODE” CASES (Submit Presentation in Portfolio).<br />
PHS NUMBER<br />
DIAGNOSIS
UTILIZED THE FOLLOWING LYMPH NODE RESOURCES<br />
<br />
Lymph node chapter in Sternberg. [Highly recommended]<br />
Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E., Pileri, S.A., Stein, H., Thiele, J., Vardiman, J.<br />
(Eds.): WHO Classification <strong>of</strong> Tumours Pathology <strong>of</strong> Haematopoietic and Lymphoid Tumours,<br />
IARC, Lyon, 2008. [Highly recommended]<br />
<br />
<br />
<br />
<br />
<br />
Checkpath (images, histories and explanations <strong>of</strong> faculty CME program for <strong>Hematopathology</strong>)<br />
(PUH G315 and in Dr. Swerdlow’s coordinator’s <strong>of</strong>fice).<br />
Teaching/conference sets <strong>of</strong> glass slides <strong>of</strong> marrows, lymph nodes, etc. (Dr. Swerdlow’s <strong>of</strong>fice).<br />
Leonard, D Diagnostic Molecular Pathology (Volume 41 in the series “Major Problems in<br />
Pathology”), Saunders, 2003.<br />
Jaffe, E.S., Harris, N.L., H. Vardiman, J.W., Campo, E., Arber, Daniel A., <strong>Hematopathology</strong>,<br />
2011 (G323)<br />
O’Malley D.P., George, T.I., Orazi A., Benign and Reactive Conditions <strong>of</strong> Lymph Node and<br />
Spleen, 2009.<br />
NOTE: Reading is an important component <strong>of</strong> this rotation. It is recognized that not all <strong>of</strong> the above<br />
resources can be used nor can most be read in entirety. Use <strong>of</strong> electronic and other resources to find<br />
and read up-to-date journal articles is also critical.<br />
I have completed the Lymph Node Checklist.<br />
Name __________________________________________ (printed)<br />
__________________________________________ (signature)<br />
Date<br />
__________________________________________
Protocol for the Examination <strong>of</strong> Specimens From Patients With<br />
Non-Hodgkin Lymphoma/Lymphoid Neoplasms<br />
Protocol applies to non-Hodgkin lymphoma/lymphoid neoplasms involving any site<br />
except the ocular adnexa, bone marrow, mycosis fungoides, and Sezary syndrome.<br />
Based on AJCC/UICC TNM, 7 th Edition<br />
Protocol web posting date: October 2013<br />
Procedures<br />
• Biopsy<br />
• Resection <strong>of</strong> Lymph Node(s) or Other Organ(s)<br />
Authors<br />
Jerry W. Hussong, MD, DDS, FCAP*<br />
<strong>Department</strong> <strong>of</strong> Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California<br />
Daniel A. Arber, MD<br />
<strong>Department</strong> <strong>of</strong> Pathology, Stanford University School <strong>of</strong> Medicine, Stanford, California<br />
Kyle T. Bradley MD, MS, FCAP<br />
<strong>Department</strong> <strong>of</strong> Pathology and Laboratory Medicine, Emory University Hospital, Atlanta, Georgia<br />
Michael S. Brown, MD, FCAP<br />
<strong>Department</strong> <strong>of</strong> Pathology, Yellowstone Pathology Institute Inc, Billings, Montana<br />
Chung-Che Chang, MD, PhD, FCAP<br />
<strong>Department</strong> <strong>of</strong> Pathology, The Methodist Hospital, Houston, Texas<br />
Monica E. de Baca, MD, FCAP<br />
<strong>Department</strong> <strong>of</strong> Pathology and Laboratory Medicine, Physicians Laboratory Ltd, Sioux Falls, South Dakota<br />
David W. Ellis, MBBS, FRCPA<br />
<strong>Department</strong> <strong>of</strong> Anatomical Pathology, Flinders Medical Centre, Bedford Park, South Australia<br />
Kathryn Foucar, MD, FCAP<br />
<strong>Department</strong> <strong>of</strong> Pathology, University <strong>of</strong> New Mexico, Albuquerque, New Mexico<br />
Eric D. Hsi, MD, FCAP<br />
<strong>Department</strong> <strong>of</strong> Clinical Pathology, Cleveland Clinic Foundation, Cleveland, Ohio<br />
Elaine S. Jaffe, MD<br />
<strong>Hematopathology</strong> Section, Laboratory <strong>of</strong> Pathology, National Cancer Institute, Bethesda, Maryland<br />
Joseph Khoury, MD, FCAP<br />
MD Anderson Cancer Center, Houston, Texas<br />
Michael Lill, MB, BS, FRACP, FRCPA<br />
<strong>Department</strong> <strong>of</strong> Medicine, Cedars-Sinai Medical Center, Los Angeles, California<br />
Stephen P. McClure, MD<br />
<strong>Department</strong> <strong>of</strong> Pathology and Laboratory Medicine, Presbyterian Pathology Group, Charlotte, North<br />
Carolina<br />
L. Jeffrey Medeiros, MD, FCAP<br />
<strong>Department</strong> <strong>of</strong> <strong>Hematopathology</strong>, MD Anderson Cancer Center, Houston, Texas<br />
Sherrie L. Perkins, MD, PhD, FCAP<br />
<strong>Department</strong> <strong>of</strong> Pathology, <strong>Hematopathology</strong>, University <strong>of</strong> Utah Health Sciences Center, Salt Lake City,<br />
Utah<br />
For the Members <strong>of</strong> the Cancer Committee, College <strong>of</strong> American Pathologists<br />
* Denotes the primary and senior author. All other contributing authors are listed alphabetically.
Surgical Pathology Cancer Case Summary<br />
Protocol web posting date: October 2013<br />
NON-HODGKIN LYMPHOMA/LYMPHOID NEOPLASMS: Biopsy, Resection<br />
Select a single response unless otherwise indicated.<br />
Specimen (select all that apply) (note A)<br />
Lymph node(s)<br />
Other (specify):<br />
Not specified<br />
Procedure<br />
Biopsy<br />
Resection<br />
Other (specify):<br />
Not specified<br />
Tumor Site (select all that apply) (note B)<br />
Lymph node(s), site not specified<br />
Lymph node(s)<br />
Specify site(s):<br />
Other tissue(s) or organ(s):<br />
Not specified<br />
Histologic Type (note C)<br />
Histologic type cannot be assessed<br />
Precursor Lymphoid Neoplasms<br />
B lymphoblastic leukemia/lymphoma, not otherwise specified (NOS) #<br />
B lymphoblastic leukemia/lymphoma with t(9;22)(q34;q11.2); BCR-ABL1<br />
B lymphoblastic leukemia/lymphoma with t(v;11q23); MLL rearranged<br />
B lymphoblastic leukemia/lymphoma with t(12;21)(p13;q22); TEL-AML1 (ETV6-RUNX1)<br />
B lymphoblastic leukemia/lymphoma with hyperdiploidy<br />
B lymphoblastic leukemia/lymphoma with hypodiploidy (hypodiploid acute lymphoblastic<br />
leukemia/lymphoma [ALL])<br />
B lymphoblastic leukemia/lymphoma with t(5;14)(q31;q32); IL3-IGH<br />
B lymphoblastic leukemia/lymphoma with t(1;19)(q23;p13.3); E2A-PBX1 (TCF3-PBX1)<br />
T lymphoblastic leukemia/lymphoma<br />
Mature B-Cell Neoplasms<br />
B-cell lymphoma, subtype cannot be determined (Note: not a category within the WHO<br />
classification)<br />
Chronic lymphocytic leukemia/small lymphocytic lymphoma<br />
B-cell prolymphocytic leukemia<br />
Splenic B-cell marginal zone lymphoma<br />
Hairy cell leukemia<br />
Splenic B-cell lymphoma/leukemia, unclassifiable
Splenic diffuse red pulp small B-cell lymphoma<br />
Hairy cell leukemia-variant<br />
Lymphoplasmacytic lymphoma<br />
Gamma heavy chain disease<br />
Mu heavy chain disease<br />
Alpha heavy chain disease<br />
Plasma cell myeloma<br />
Solitary plasmacytoma <strong>of</strong> bone<br />
Extraosseous plasmacytoma<br />
Extranodal marginal zone lymphoma <strong>of</strong> mucosa-associated lymphoid tissue (MALT lymphoma)<br />
Nodal marginal zone lymphoma<br />
Pediatric nodal marginal zone lymphoma<br />
Follicular lymphoma<br />
Pediatric follicular lymphoma<br />
Primary intestinal follicular lymphoma<br />
Primary cutaneous follicle center lymphoma<br />
Mantle cell lymphoma<br />
Diffuse large B-cell lymphoma (DLBCL), NOS<br />
T cell/histiocyte-rich large B-cell lymphoma<br />
Primary DLBCL <strong>of</strong> the central nervous system (CNS)<br />
Primary cutaneous DLBCL, leg type<br />
Epstein-Barr virus (EBV)-positive DLBCL <strong>of</strong> the elderly<br />
DLBCL associated with chronic inflammation<br />
Lymphomatoid granulomatosis<br />
Primary mediastinal (thymic) large B-cell lymphoma<br />
Intravascular large B-cell lymphoma<br />
Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma<br />
Plasmablastic lymphoma<br />
Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease<br />
Primary effusion lymphoma<br />
Burkitt lymphoma<br />
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma<br />
and Burkitt lymphoma<br />
B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma<br />
and classical Hodgkin lymphoma<br />
Other (specify):<br />
Mature T- and NK-Cell Neoplasms<br />
T-cell lymphoma, subtype cannot be determined (Note: not a category within the WHO<br />
classification)<br />
T-cell prolymphocytic leukemia<br />
T-cell large granular lymphocytic leukemia<br />
Chronic lymphoproliferative disorder <strong>of</strong> NK cells<br />
Aggressive NK-cell leukemia<br />
Systemic EBV-positive T-cell lymphoproliferative disease <strong>of</strong> childhood<br />
Hydroa vacciniforme-like lymphoma<br />
Adult T-cell leukemia/lymphoma<br />
Extranodal NK/T-cell lymphoma, nasal type<br />
Enteropathy-associated T-cell lymphoma<br />
Hepatosplenic T-cell lymphoma<br />
Subcutaneous panniculitis-like T-cell lymphoma<br />
Primary cutaneous anaplastic large cell lymphoma
Lymphomatoid papulosis<br />
Primary cutaneous gamma-delta T-cell lymphoma<br />
Primary cutaneous CD8-positive aggressive epidermotropic cytotoxic T-cell lymphoma<br />
Primary cutaneous CD4-positive small/medium T-cell lymphoma<br />
Peripheral T-cell lymphoma, NOS<br />
Angioimmunoblastic T-cell lymphoma<br />
Anaplastic large cell lymphoma, ALK-positive<br />
Anaplastic large cell lymphoma, ALK-negative<br />
Other (specify):<br />
Histiocytic and Dendritic Cell Neoplasms<br />
Histiocytic sarcoma<br />
Langerhans cell histiocytosis<br />
Langerhans cell sarcoma<br />
Interdigitating dendritic cell sarcoma<br />
Follicular dendritic cell sarcoma<br />
Fibroblastic reticular cell tumor<br />
Indeterminate dendritic cell tumor<br />
Disseminated juvenile xanthogranuloma<br />
Posttransplant Lymphoproliferative Disorders (PTLD) ##<br />
Early lesions:<br />
Plasmacytic hyperplasia<br />
Infectious mononucleosis-like PTLD<br />
Polymorphic PTLD<br />
Monomorphic PTLD (B- and T/NK-cell types)<br />
Specify subtype:<br />
Classical Hodgkin lymphoma type PTLD ###<br />
Note: Italicized histologic types denote provisional entities in the 2008 WHO classification.<br />
#<br />
An initial diagnosis <strong>of</strong> “B lymphoblastic leukemia/lymphoma, NOS” may need to be given before the cytogenetic<br />
results are available.<br />
##<br />
These disorders are listed for completeness, but not all <strong>of</strong> them represent frank lymphomas.<br />
###<br />
Classical Hodgkin lymphoma type PTLD can be reported using either this protocol or the separate College <strong>of</strong><br />
American Pathologists protocol for Hodgkin lymphoma. 1<br />
+ Pathologic Extent <strong>of</strong> Tumor (select all that apply) (note D)<br />
+ Bone marrow involvement<br />
+ Other site involvement<br />
+ Specify site(s):<br />
+ Additional Pathologic Findings<br />
+ Specify:<br />
Immunophenotyping (flow cytometry and/or immunohistochemistry) (note E)<br />
Performed, see separate report:<br />
Performed<br />
Specify method(s) and results:<br />
Not performed
+ Cytogenetic Studies (note E)<br />
+ Performed, see separate report:<br />
+ Performed<br />
+ Specify method(s) and results:<br />
+ Not performed<br />
+ Molecular Genetic Studies (note E)<br />
+ Performed, see separate report:<br />
+ Performed<br />
+ Specify method(s) and results:<br />
+ Not performed<br />
+ Clinical Prognostic Factors and Indices (select all that apply) (note F)<br />
+ International Prognostic Index (IPI) (specify):<br />
+ Follicular Lymphoma International Prognostic Index (FLIPI) (specify):<br />
+ B symptoms present<br />
+ Other (specify):<br />
+ Comment(s)<br />
+ Data elements preceded by this symbol are not required. However, these elements may be clinically important but are not yet<br />
validated or regularly used in patient management.
Explanatory Notes<br />
A. Specimen<br />
Any number <strong>of</strong> specimen types may be submitted in the evaluation <strong>of</strong> lymphoid neoplasms. Lymph<br />
nodes, skin, gastrointestinal (GI) tract, bone marrow, spleen, thymus, and tonsils are among the most<br />
common. Specimens submitted with a suspected diagnosis <strong>of</strong> lymphoma require special handling in<br />
order to optimize the histologic diagnosis and to prepare the tissue for molecular and other ancillary<br />
special studies. 2,3 The guidelines detailed below are suggested for specimen handling in cases <strong>of</strong><br />
suspected lymphoma.<br />
• Tissue should be received fresh. Unsectioned lymph nodes should not be immersed in fixative, and<br />
care should be taken to make thin slices <strong>of</strong> the node to ensure optimal penetration <strong>of</strong> fixative.<br />
• The fresh specimen size, color, and consistency should be recorded, as should the presence or<br />
absence <strong>of</strong> any visible nodularity, hemorrhage, or necrosis after serial sectioning at 2-mm intervals<br />
perpendicular to the long axis <strong>of</strong> the lymph node.<br />
• Touch imprints may be made from the freshly cut surface, and the imprints fixed in alcohol or air<br />
dried.<br />
• For cytogenetic studies or culture <strong>of</strong> microorganisms: submit a fresh portion <strong>of</strong> the node (or other<br />
specimen type) sterilely in appropriate medium.<br />
• For immunophenotyping by flow cytometry: submit a fresh portion <strong>of</strong> the specimen in appropriate<br />
transport medium such as RPMI.<br />
• Fixation (record fixative[s] used for individual slices <strong>of</strong> the specimen):<br />
o Estimated time from excision to fixation should be noted, if possible, as this may impact<br />
preservation or recovery <strong>of</strong> certain analytes such as RNA and phosphoproteins in fixed tissues.<br />
o Zinc formalin or B5 produces superior cytologic detail but is not suitable for DNA extraction and<br />
may impair some immunostains (eg, CD30). B5 also has the additional limitation <strong>of</strong> requiring<br />
proper hazardous-materials disposal.<br />
o Formalin fixation is preferable when the tissue sample is limited, as it is most suitable for many<br />
ancillary tests such as molecular/genetic studies, in-situ hybridization, and immunophenotyping.<br />
o Over-fixation (ie, more than 24 hours in formalin, more than 4 hours in zinc formalin or B5) should<br />
be avoided for optimal immunophenotypic reactivity.<br />
• Snap-frozen tissue is optimal for DNA and RNA extraction.<br />
o Place in aluminum foil or cover in OCT.<br />
o Immerse in dry ice/isopentane slush or liquid nitrogen.<br />
o Store at -80°C until needed.<br />
B. Tumor Site<br />
The anatomic sites that constitute the major structures <strong>of</strong> the lymphatic system include groups and<br />
chains <strong>of</strong> lymph nodes, the spleen, the thymus, Waldeyer’s ring (a circular band <strong>of</strong> lymphoid tissue that<br />
surrounds the oropharynx, consisting <strong>of</strong> the palatine, lingual, and pharyngeal tonsils), the vermiform<br />
appendix, and the Peyer’s patches <strong>of</strong> the ileum. 2,3 Minor sites <strong>of</strong> lymphoid tissue include the bone<br />
marrow, mediastinum, liver, skin, lung, pleura, and gonads. Involvement <strong>of</strong> extranodal sites is more<br />
common in non-Hodgkin lymphomas (NHL) than in Hodgkin lymphoma. In addition, some NHL, such as<br />
mycosis fungoides and extranodal marginal zone lymphoma <strong>of</strong> mucosa-associated lymphoid tissue<br />
(MALT), occur predominantly or entirely in extranodal sites.<br />
C. Histologic Type<br />
This protocol recommends assigning histologic type based on the World Health Organization (WHO)<br />
classification <strong>of</strong> lymphoid neoplasms. 4 It was originally published in 2001 and recently was revised and<br />
updated in 2008. 5 This classification encompasses both nodal and extranodal lymphomas and provides<br />
distinction <strong>of</strong> individual lymphoid neoplasms based upon morphologic, immunophenotypic,<br />
cytogenetic, and clinical features. While histologic examination typically is the gold standard, the<br />
majority <strong>of</strong> the lymphoid neoplasms will require the utilization <strong>of</strong> 1 or more other ancillary techniques,
such as immunophenotyping, molecular studies, and/or cytogenetics, to arrive at the correct<br />
diagnosis. 4-10 If the specimen is inadequate or suboptimal for a definitive diagnosis and subtyping, this<br />
information should also be relayed to the clinician with an explanation <strong>of</strong> what makes the specimen<br />
inadequate or suboptimal.<br />
D. Pathologic Extent <strong>of</strong> Tumor (Stage)<br />
In general, the TNM classification has not been used for staging <strong>of</strong> lymphomas because the site <strong>of</strong> origin<br />
<strong>of</strong> the tumor is <strong>of</strong>ten unclear and there is no way to differentiate among T, N, and M. Thus, a special<br />
staging system (Ann Arbor system) is used for both Hodgkin lymphoma and NHL. 11,12 It was originally<br />
published over 30 years ago for staging Hodgkin lymphoma. The Ann Arbor classification for<br />
lymphomas has been applied to NHL by the American Joint Committee on Cancer (AJCC) 13 and the<br />
International Union Against Cancer (UICC) except for mycosis fungoides and Sezary syndrome. 14<br />
For multiple myeloma, the Durie-Salmon staging system is recommended by the AJCC. 13-15 The<br />
international staging system for multiple myeloma is useful for determining survival. 13 The Ann Arbor<br />
classification and Durie-Salmon staging systems are shown below. It should also be realized that the St.<br />
Jude staging system is commonly used for pediatric patients. 16<br />
Historically, pathologic staging depended on the biopsy <strong>of</strong> multiple lymph nodes on both sides <strong>of</strong> the<br />
diaphragm, splenectomy, wedge liver biopsy, and bone marrow biopsy to assess distribution <strong>of</strong> disease.<br />
Currently, staging <strong>of</strong> NHL is more commonly clinical than pathologic. Clinical staging generally involves<br />
a combination <strong>of</strong> clinical, radiologic, and surgical data. Physical examination, laboratory tests (eg,<br />
complete blood examination and blood chemistry studies including lactate dehydrogenase [LDH] and<br />
liver function tests), imaging studies (eg, computed tomography scans, magnetic resonance imaging<br />
studies, and positron emission tomography), biopsy (to determine diagnosis, histologic type, and extent<br />
<strong>of</strong> disease), and bone marrow examination are <strong>of</strong>ten required. In patients at high risk for occult CNS<br />
involvement, cerebrospinal fluid cytology should be performed.<br />
There is almost universal agreement that the stage <strong>of</strong> the NHL is prognostically significant. 17-20 Correct<br />
diagnosis and staging are the key factors in National Comprehensive Cancer Network treatment<br />
schema that most clinicians utilize. 21<br />
AJCC/UICC Staging for Non-Hodgkin Lymphomas<br />
Stage I Involvement <strong>of</strong> a single lymph node region (I), or localized involvement <strong>of</strong> a single<br />
extralymphatic organ or site in the absence <strong>of</strong> any lymph node involvement (IE) # , ##<br />
Stage II Involvement <strong>of</strong> 2 or more lymph node regions on the same side <strong>of</strong> the diaphragm (II), or<br />
localized involvement <strong>of</strong> a single extralymphatic organ or site in association with regional<br />
lymph node involvement with or without involvement <strong>of</strong> other lymph node regions on<br />
the same side <strong>of</strong> the diaphragm (IIE) ## , ###<br />
Stage III Involvement <strong>of</strong> lymph node regions on both sides <strong>of</strong> the diaphragm (III), which also may<br />
be accompanied by extralymphatic extension in association with adjacent lymph node<br />
involvement (IIIE) or by involvement <strong>of</strong> the spleen (IIIS) or both (IIIE+S) ## , ### ,^<br />
Stage IV Diffuse or disseminated involvement <strong>of</strong> 1 or more extralymphatic organs, with or without<br />
associated lymph node involvement; or isolated extralymphatic organ involvement in<br />
the absence <strong>of</strong> adjacent regional lymph node involvement, but in conjunction with<br />
disease in distant site(s). Stage IV includes any involvement <strong>of</strong> the liver, bone marrow, or<br />
nodular involvement <strong>of</strong> the lung(s) or cerebral spinal fluid. ## , ### ,^<br />
# Multifocal involvement <strong>of</strong> a single extralymphatic organ is classified as stage IE and not stage IV.<br />
## For all stages, tumor bulk greater than 10 to 15 cm is an unfavorable prognostic factor.
### The number <strong>of</strong> lymph node regions involved may be indicated by a subscript: eg, II 3 . For stages II to<br />
IV, involvement <strong>of</strong> more than 2 sites is an unfavorable prognostic factor.<br />
^ For stages III to IV, a large mediastinal mass is an unfavorable prognostic factor.<br />
Note: Direct spread <strong>of</strong> a lymphoma into adjacent tissues or organs does not influence classification <strong>of</strong><br />
stage.<br />
AJCC/UICC Staging for Plasma Cell Myeloma<br />
Stage I Hemoglobin greater than 10.0 g/dL<br />
Serum calcium 12 mg/dL or less<br />
Normal bone x-rays or a solitary bone lesion<br />
IgG less than 5 g/dL<br />
IgA less than 3 g/dL<br />
Urine M-protein less than 4 g/24 hours<br />
Stage III One or more <strong>of</strong> the following are included:<br />
Hemoglobin less than 8.5 g/dL<br />
Serum calcium greater than 12 mg/dL<br />
Advanced lytic bone lesions<br />
IgG greater than 7 g/dL<br />
IgA greater than 5 g/dL<br />
Urine M-protein greater than 12 g/24 hours<br />
Stage II Disease fitting neither stage I nor stage III<br />
Note: Patients are further classified as (A) serum creatinine less than 2.0 mg/dL or (B) serum creatinine<br />
2.0 mg/dL or greater. The median survival for stage IA disease is about 5 years, and that for stage IIIB<br />
disease is 15 months. 13,14<br />
E. Immunophenotyping and Molecular Genetic Studies<br />
Immunophenotyping can be performed by flow cytometry 8 or immunohistochemistry. Each has its<br />
advantages and disadvantages. Flow cytometry is rapid (hours), quantitative, and allows multiple<br />
antigens to be evaluated on the same cell simultaneously. Antigen positivity, however, cannot be<br />
correlated with architecture or cytologic features. Immunohistochemistry requires hours/days to<br />
perform, quantitation is subjective, but importantly it allows correlation <strong>of</strong> antigen expression with<br />
architecture and cytology. Not all antibodies are available for immunohistochemistry, particularly in<br />
fixed tissues, but one <strong>of</strong> its advantages is that it can be performed on archival tissue. Both techniques<br />
can provide diagnostic as well as clinically relevant information (eg, identification <strong>of</strong> therapeutic targets<br />
such as CD20). Molecular studies now play an increasingly important role in the diagnosis <strong>of</strong><br />
hematopoietic neoplasms. They aid not only in helping establish clonality but also in determining<br />
lineage, establishing the diagnosis <strong>of</strong> specific disease entities, and monitoring minimal residual<br />
disease. 10,22-24<br />
Immunophenotypes and Genetics<br />
The following is to be used as a guideline for the more common immunophenotyping and cytogenetic<br />
findings for each entity. 3,4,8,22-27 It is however, not entirely comprehensive and individual cases may vary<br />
somewhat in their immunophenotypic and cytogenetic pr<strong>of</strong>ile.<br />
Precursor Lymphoid Neoplasms<br />
B Lymphoblastic Leukemia/Lymphoma, NOS: sIG-, cytoplasmic µ chain (30%), CD19+, CD20-/+, CD22+,<br />
PAX5+, CD79a+, TdT+, HLA-DR+, CD10+/-, CD34+/-, CD13-/+, CD33-/+, IGH gene rearrangement +/-, IGL<br />
gene rearrangement -/+, TCR gene rearrangement -/+, variable cytogenetic abnormalities
B Lymphoblastic Leukemia/Lymphoma With t(9;22)(q34;q11.2); BCR-ABL1: sIG-, cytoplasmic µ chain<br />
(30%), CD19+, CD20-/+, CD22+, PAX5+, CD79a+, TdT+, HLA-DR+, CD10+/-, CD34+/-, CD13-/+, CD33-/+,<br />
IGH gene rearrangement +/-, IGL gene rearrangement -/+, TCR gene rearrangement -/+,<br />
t(9;22)(q34;q11.2), may have either p190 kd or p210 kd BCR-ABL1 fusion protein.<br />
B Lymphoblastic Leukemia/Lymphoma With t(v;11q23); MLL Rearranged: sIG-, cytoplasmic µ chain<br />
(30%), CD19+, CD20-/+, CD22+, PAX5+, CD79a+, TdT+, HLA-DR+, CD10-, CD34+/-, CD13-/+, CD33-/+,<br />
CD15 +/-, IGH gene rearrangement +/-, IGL gene rearrangement -/+, TCR gene rearrangement -/+,<br />
t(v;11q23)<br />
B Lymphoblastic Leukemia/Lymphoma With t(12;21)(p13;q22); TEL-AML1 (ETV6-RUNX1): sIG-, cytoplasmic<br />
µ chain (30%), CD19+, CD20-, CD22+, PAX5+, CD79a+, TdT+, HLA-DR+, CD10+, CD34+/-, CD13+/-, CD33-<br />
/+, CD15 +/-, IGH gene rearrangement +/-, IGL gene rearrangement -/+, TCR gene rearrangement -/+,<br />
t(12;21)(p13;q22)<br />
B Lymphoblastic Leukemia/Lymphoma With Hyperdiploidy: sIG-, cytoplasmic µ chain (30%), CD19+,<br />
CD20-/+, CD22+, PAX5+, CD79a+, TdT+, HLA-DR+, CD10+/-, CD34+/-, CD13-/+, CD33-/+, IGH gene<br />
rearrangement +/-, IGL gene rearrangement -/+, TCR gene rearrangement -/+, hyperdiploid (>50<br />
chromosomes, <strong>of</strong>ten with extra copies <strong>of</strong> chromosomes 21, X, 4 and 14) without structural abnormalities<br />
B Lymphoblastic Leukemia/Lymphoma With Hypodiploidy: sIG-, cytoplasmic µ chain (30%), CD19+,<br />
CD20-/+, CD22+, PAX5+, CD79a+, TdT+, HLA-DR+, CD10+/-, CD34+/-, CD13-/+, CD33-/+, IGH gene<br />
rearrangement +/-, IGL gene rearrangement -/+, TCR gene rearrangement -/+, hypodiploid with 45<br />
chromosomes to near haploid<br />
B Lymphoblastic Leukemia/Lymphoma With t(5;14)(q31;q32); IL3-IGH: sIG-, cytoplasmic µ chain (30%),<br />
CD19+, CD20-, CD22+, PAX5+, CD79a+, TdT+, HLA-DR+, CD10+, CD34+/-, CD13+/-, CD33-/+, CD15 +/-,<br />
IGH gene rearrangement +/-, IGL gene rearrangement -/+, TCR gene rearrangement -/+,<br />
t(5:14)(q31;q32)<br />
B Lymphoblastic Leukemia/Lymphoma With t(1;19)(q23;p13.3); E2A-PBX1 (TCF3-PBX1): sIG-, cytoplasmic<br />
µ chain (30%), CD19+, CD20-, CD22+, PAX5+, CD79a+, TdT+, HLA-DR+, CD10+, CD34+/-, CD13+/-, CD33-<br />
/+, CD15 +/-, IGH gene rearrangement +/-, IGL gene rearrangement -/+, TCR gene rearrangement -/+,<br />
t(1;19)(q23;p13.3)<br />
T Lymphoblastic Leukemia/Lymphoma: TdT+, CD7+, CD3+/- (usually surface CD3-), variable expression<br />
<strong>of</strong> other PanT antigens, CD1a+/-, <strong>of</strong>ten CD4 and CD8 double positive or double negative, IG-, PanB-;<br />
variable TCR gene rearrangements; IGH gene rearrangement -/+, chromosomal abnormalities are<br />
common and <strong>of</strong>ten involve 14q11-14, 7q35, or 7p14-15<br />
Mature B-Cell Neoplasms<br />
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: Faint sIGM+, sIGD+/-,<br />
cIG-/+, panB+ (CD19+, CD20+), CD5+, CD10-, CD23+, CD43+, CD11c-/+; IGH and IGL gene<br />
rearrangements; trisomy 12; del 13q, del(17p), or del(11q) can be seen<br />
B-Cell Prolymphocytic Leukemia: sIgM, sIgD+/-, pan B+ (CD19, CD20, CD22, CD79a, CD79b and FMC-7),<br />
CD5 -/+, CD23-/+, del(17p), t(11;14)(q13;q32), breakpoints involving 13q14<br />
Splenic B-Cell Marginal Zone Lymphoma: sIGM+, sIGD+/-, CD20+, CD79a+, CD5-, CD10-, CD23-, CD43-,<br />
nuclear cyclin D1-, CD103-, allelic loss at 7q31-32 (40%)
Hairy Cell Leukemia: sIG+ (IGM, IGD, IGG, or IGA), PanB+, CD79a+, CD79b-, DBA.44+, CD123+, CD5-,<br />
CD10-, CD23-, CD11c+, CD25+, FMC7+, CD103+ (mucosal lymphocyte antigen as detected by B-ly7),<br />
tartrate resistant acid phosphatase (TRAP)+; IGH and IGL gene rearrangements, no specific cytogenetic<br />
findings<br />
Splenic Diffuse Red Pulp Small B-Cell Lymphoma: sIGG+, sIGD-/+, sIGM+/-, CD20+, DBA.44+, CD5-,<br />
CD103-/+, CD123-, CD25-, CD11c-/+, CD10-, CD23-, t(9;14)(p13;q32) occasionally seen, rarely<br />
abnormalities in TP53 or del 7q<br />
Hairy Cell Leukemia-Variant: sIGG+, PanB+, DBA.44+, CD11c+, CD103+, FMC7+, CD25-, CD123-, Annexin<br />
A1-, TRAP-IHC-, no specific cytogenetic findings<br />
Lymphoplasmacytic Lymphoma: sIGM+, sIGD-/+, cIG+, PanB+, CD19+, CD20+, CD138+ (in plasma<br />
cells), CD79a+, CD5-, CD10-, CD43+/-, CD25-/+; IGH and IGL gene rearrangements, no specific<br />
cytogenetic findings<br />
Alpha Heavy Chain Disease (Immunoproliferative Small Intestinal Disease): cytoplasmic alpha heavy<br />
chain+, CD20+ (lymphocytes), CD138+ (plasma cells), light chain-<br />
Gamma Heavy Chain Disease: IgG heavy chain+, CD79a+, CD20+ (on lymphocytes), CD138+ (in<br />
plasma cells), CD5-, CD10-, light chain-, abnormal karyotype in 50% without recurring abnormalities<br />
Mu Heavy Chain Disease: monoclonal cytoplasmic mu heavy chain+, B-cell antigen+, CD5-, CD10-,<br />
surface light chain-<br />
Plasma Cell Myeloma: cIG+ (IGG, IGA, rare IGD, IGM, or IGE or light chain only), PanB-(CD19-, CD20-,<br />
CD22-), CD79a+/-, CD45-/+, HLA-DR-/+, CD38+, CD56+/-, CD138+, EMA-/+, CD43+/-, cyclin D1+; IGH<br />
and IGL gene rearrangements; numerical and structural chromosomal abnormalities are common,<br />
including trisomies (<strong>of</strong>ten involving odd numbered chromosomes), deletions (most commonly involving<br />
13q14), and translocations (<strong>of</strong>ten involving 14q32)<br />
Solitary Plasmacytoma <strong>of</strong> Bone: cIG+ (IGG, IGA, rare IGD, IGM, or IGE or light chain only), PanB-(CD19-,<br />
CD20-, CD22-), CD79a+/-, CD45-/+, HLA-DR-/+, CD38+, CD56+/-, CD138+, EMA-/+, CD43+/-, cyclin D1+;<br />
IGH and IGL gene rearrangements; deletions, most commonly 13q, and occasional translocations, in<br />
particular t(11;14)(q13;q32)<br />
Extraosseous Plasmacytoma: cIG+ (IGG, IGA, rare IGD, IGM, or IGE or light chain only), PanB-(CD19-,<br />
CD20-, CD22-), CD79a+/-, CD45-/+, HLA-DR-/+, CD38+, CD56+/-, CD138+, EMA-/+, CD43+/-, cyclin D1+;<br />
IGH and IGL gene rearrangements; deletions, most commonly 13q, and occasional translocations, in<br />
particular t(11;14)(q13;q32)<br />
Extranodal Marginal Zone Lymphoma <strong>of</strong> Mucosa-Associated Lymphoid Tissue<br />
(MALT Lymphoma): sIG+ (IGM or IGA or IGG), sIGD-, cIG-/+, PanB+, CD5-, CD10-, CD23-, CD43-/+; IGH<br />
and IGL gene rearrangements, BCL1 and BCL2 germline, trisomy 3 or t(11;18)(q21;q21) may be seen<br />
Nodal Marginal Zone Lymphoma: sIGM+, sIGD-, cIG-/+, PanB+, CD5-, CD10-, CD23-, CD43-/+; IGH and<br />
IGL gene rearrangements, BCL1 and BCL2 germline<br />
Follicular Lymphoma: sIG+ (usually IGM +/- IGD, IGG, IGA), PanB+, CD10+/-, CD5-/+, CD23-/+, CD43-,<br />
CD11c-, CD25-; overexpression <strong>of</strong> BCL2+ (useful to distinguish from reactive follicles), BCL6+; IGH and IGL<br />
gene rearrangements, t(14;18)(q32;q21) with rearranged BCL2 gene (70-95% in adults)
Pediatric Follicular Lymphoma: sIG+ (usually IGM +/- IGD, IGG, IGA), PanB+, CD10+/-, CD5-, CD23-/+,<br />
CD43-, CD11c-, CD25-; overexpression <strong>of</strong> BCL2-, BCL6+, t(14;18) with rearranged BCL2 gene -<br />
Primary Cutaneous Follicle Center Lymphoma: CD20+, CD79a+, CD10+/-, BCL2-/+, BCL6+, CD5-, CD43-,<br />
BCL2 gene rearrangement-/+<br />
Mantle Cell Lymphoma: sIGM+, sIGD+, lambda>kappa, PanB+, CD5+, CD10-/+, CD23-, CD43+, CD11c-,<br />
CD25-, cyclin D1+; IGH and IGL gene rearrangements, t(11;14)(q13;q32); BCL1 gene rearrangements<br />
(CCND1/cyclinD1) common<br />
Diffuse Large B-Cell Lymphoma (DLBCL), NOS: PanB+, surface or cytoplasmic IGM>IGG>IGA, CD45+/-,<br />
CD5-/+, CD10+/-, BCL6 +/-, 3q27 region abnormalities involving BCL6 seen in 30% <strong>of</strong> cases, t(14;18)<br />
involving BCL2 seen in 20-30% <strong>of</strong> cases, MYC rearrangement seen in 10% <strong>of</strong> cases<br />
T Cell/Histiocytic-Rich Large B-Cell Lymphoma: PanB+, BCL6+, BCL2-/+, EMA -/+, background comprised<br />
<strong>of</strong> CD3 and CD5 positive T-cells and CD68+ histiocytes<br />
Primary DLBCL <strong>of</strong> the CNS: CD20+, CD22, CD79a, CD10-/+, BCL6+/-, IRF4/MUM1+/-, BCL2+/-, BCL6<br />
translocations+/-, del 6q and gains <strong>of</strong> 12q, 22q, and 18q21 common<br />
Primary Cutaneous DLBCL, Leg Type: sIG+, CD20+, CD79a+, CD10-, BCL2+, BCL6+, IRF4/MUM1+, FOX-<br />
P1+; translocations involving MYC, BCL6, and IGH genes are common<br />
EBV-Positive Diffuse Large B-Cell Lymphoma <strong>of</strong> the Elderly: CD20+/-, CD79a+/-, CD10-, IRF4/MUM1+/-,<br />
BCL6-, LMP+, EBER+<br />
DLBCL Associated With Chronic Inflammation: CD20+/-, CD79a+/-, CD138-/+, IRF4/MUM1-/+, CD30-/+, T-<br />
cells markers-/+, LMP+/-, EBER+/-<br />
Lymphomatoid Granulomatosis: CD20+, CD30+/-, CD79a-/+, CD15-, LMP+/-, EBER+.<br />
Primary Mediastinal (Thymic) Large B-Cell Lymphoma: sIG-/+, PanB+, (especially CD20, CD79a),<br />
CD45+/-, CD15-, CD30-/+ (weak), IRF4/MUM1 +/-, BCL2+/-, BCL6+/-, CD23+, MAL+; IGH and IGL gene<br />
rearrangements<br />
Intravascular Large B-Cell Lymphoma: Pan B+ (CD19, CD20, CD22, CD79a), CD5-/+, CD10-/+,<br />
IRF4/MUM1+<br />
ALK-Positive Large B-Cell Lymphoma: ALK+, CD138+, EMA+, VS38+, CD45-/+, CD4-/+,<br />
CD57-/+, CD20-, CD79a-, CD3-, CD30-/+, IRF4/MUM1-/+, t(2;17)(p23;q23)+/-, t(2;5)(p23;35)-/+<br />
Plasmablastic Lymphoma: CD38+, CD138+, Vs38c+, IRF4/MUM1+, CD79a+/-, EMA +/-, CD30+/-, CD45-/+,<br />
CD20-/+, PAX5-/+, EBER+/-, EMA+/-, CD30+/-<br />
Large B-Cell Lymphoma Arising in HHV8-Associated Multicentric Castleman Disease: CD20+/-, CD79a-,<br />
CD38-/+, CD138-, EBER-, lambda light chain restricted<br />
Primary Effusion Lymphoma: CD45+/-, CD30+/-, CD38+/-, CD138+/-, EMA+/-, CD19-, CD20-, CD79a-,<br />
CD3-/+, BCL6-, HHV8/KSHV+, EBV+/-, IGH and IGL gene rearrangements<br />
Burkitt Lymphoma: sIGM+, PanB+, CD5-, CD10+, BCL6+, CD38+, CD77+, CD43+, CD23-; Ki-67 (95-100%),<br />
BCL2-; TdT-, IGH and IGL gene rearrangements, t(8;14)(q24;q32) and variants t(2;8)(p12;q24) and
t(8;22)(q24;q11); rearranged MYC gene; EBV common (95%) in endemic cases and infrequent (15-20%)<br />
in sporadic cases, intermediate incidence (30-40%) in HIV-positive cases<br />
B-Cell Lymphoma, Unclassifiable, With Features Intermediate Between Diffuse Large B-cell Lymphoma<br />
and Burkitt Lymphoma: PanB+, CD10+, BCL6+, BCL2-/+, IRF4/MUM1-, Ki-67 (50-100%), 8q24/MYC<br />
translocation (35-50%), BCL2 translocation (15%), and occasionally both translocations (so called double<br />
hit lymphoma)<br />
B-Cell Lymphoma, Unclassifiable, With Features Intermediate Between Diffuse Large B-cell Lymphoma<br />
and Classical Hodgkin Lymphoma: CD45+/-, CD20+/-, CD79a+/-, CD30+/-, CD15+/-, PAX-5+/-, OCT-2+/-,<br />
BOB.1+/-, CD10-, ALK-<br />
Mature T-Cell and NK-Cell Neoplasms<br />
T-Cell Prolymphocytic Leukemia: PanT+ (CD2, CD3, CD5, CD7), CD25-, CD4+/CD8->CD4+/CD8+>CD4-<br />
/CD8-, TCL1+, TdT-, CD1a-; TCR gene rearrangements, 75% show inv 14 with breakpoints at q11 and q32,<br />
10% have a reciprocal tandem translocation t(14;14)(q11;q32)<br />
T-Cell Large Granular Lymphocytic Leukemia: PanT+ (CD2, CD3+, CD5+/-), CD7-, TCR+, CD4-, CD8+,<br />
CD16+, CD56-, CD57+, CD25-, TIA1+, granzyme B+, TdT-; most cases show clonal TCR gene<br />
rearrangements<br />
Chronic Lymphoproliferative Disorder <strong>of</strong> NK Cells: sCD3-, cCD3+, CD16+, CD56 (weak), TIA1+, granzyme<br />
B+, CD8+/-, CD2-/+, CD7-/+, CD57-/+, EBV-, karyotype is typically normal<br />
Aggressive NK-Cell Leukemia: CD2+, sCD3-, cCD3+, CD56+, TIA+/-, CD16+/-, CD57-, Fas ligand+, EBV+,<br />
del(6)(q21q25) and del(11q) can be seen<br />
Systemic EBV-Positive T-Cell Lymphoproliferative Disease <strong>of</strong> Childhood: CD2+, CD3+, TIA+, CD8+ (if<br />
associated with acute EBV infection), EBER+, CD56-, TCR gene rearrangements+<br />
Hydroa Vacciniforme-like Lymphoma: Cytotoxic T-cell or less <strong>of</strong>ten CD56+ NK-cell phenotype, EBER+/-,<br />
TCR gene rearrangement+<br />
Adult T-Cell Leukemia/Lymphoma (HTLV1+): PanT+ (CD2+, CD3+, CD5+), CD7-, CD4+, CD8-, CD10+,<br />
CD25+, TdT-; TCR gene rearrangements, clonally integrated HTLV1<br />
Extranodal NK/T-Cell Lymphoma: CD2+, CD5-/+, CD7-/+, CD3-/+, granzyme B+, TIA1+, CD4-, CD8-,<br />
CD56+/-, TdT-; usually no TCR or Ig gene rearrangements; usually EBV positive<br />
Enteropathy-associated T-cell Lymphoma: CD3+, CD7+, CD4-, CD8-/+, CD103+, TdT-<br />
Hepatosplenic T-cell Lymphoma: CD2+, CD3+, TCR gamma-delta+, TCR alpha-beta rarely +, CD5-,<br />
CD7+, CD4-, CD8-/+, CD56+/-, CD25-; TCRG gene rearrangements +/-, variable TCRB gene<br />
rearrangements -/+; isochromosome 7q and trisomy 8 common<br />
Subcutaneous Panniculitis-like T-Cell Lymphoma: CD8+, granzyme B+, TIA1+, perforin+, TCR alpha/<br />
beta +, CD4-, CD56-<br />
Lymphomatoid Papulosis: CD4+, CD2-/+, CD3+, CD5-/+, TIA1+, granzyme B+/-, CD30+/-; TCR gene<br />
rearrangements+/-
Primary Cutaneous Anaplastic Large-Cell Lymphoma: CD4+, TIA1+/-, granzyme B+/-, perforin+/-, CD30+,<br />
CD2-/+, CD5-/+, CD3-/+, CLA+, ALK-, EMA-/+; TCR gene rearrangements+/-<br />
Primary Cutaneous Gamma-Delta T-Cell Lymphoma: TCR gamma/delta+, CD2+, CD3+, CD5-, CD56+,<br />
CD7+/-, CD4-, CD8-/+, Beta F1-<br />
Primary Cutaneous CD8-positive Aggressive Epidermotropic Cytotoxic T-cell Lymphoma: CD3+, CD8+,<br />
granzyme B+, perforin+, TIA1+, CD45RA+/-, CD2-/+, CD4-, CD5-, CD7-, EBV-, Beta F1+; TCR gene<br />
rearrangements (alpha/beta)+<br />
Primary Cutaneous CD4-Positive Small/Medium T-Cell Lymphoma: CD3+, CD4+, CD8-, CD30-, TCR gene<br />
rearrangements+<br />
Peripheral T-Cell Lymphoma, NOS: PanT variable (CD2+/-, CD3+/-, CD5-/+, CD7-/+), most cases CD4+,<br />
some cases CD8+, a few cases are CD4-/CD8-, or CD4+/CD8+; TCR gene rearrangements+<br />
Angioimmunoblastic T-Cell Lymphoma: PanT+ (<strong>of</strong>ten with variable loss <strong>of</strong> some PanT antigens), usually<br />
CD4+, PD1+, CXCL13+; TCR gene rearrangements in 75%; IGH gene rearrangements in up to 30%, EBV<br />
<strong>of</strong>ten positive in B-cells<br />
Anaplastic Large Cell Lymphoma, ALK Positive: CD30+, ALK+, EMA+/-, CD3-/+, CD2+/-, CD4+/-, CD5+/-,<br />
CD8-/+, CD43+/-, CD25+, CD45+/-, CD45RO+/-, TIA1+/-, granzyme+/-, perforin+/-, EBV-, TCR gene<br />
rearrangements+/-, t(2;5)(p23;35) in 80% <strong>of</strong> cases, t(1;2)(q25;p23) in 10-15% <strong>of</strong> cases. Other various<br />
translocations can also be seen.<br />
Anaplastic Large Cell Lymphoma, ALK Negative: CD30+ (strong/intense staining),<br />
CD2+/-, CD3+/-, CD5-/+, CD4+/-, CD8-/+, CD43+, TIA1+/-, granzyme B+/-, perforin +/-, ALK-, TCR gene<br />
rearrangements+<br />
Histiocytic and Dendritic Cell Neoplasms<br />
Histiocytic Sarcoma: CD45+, CD163+, CD68+, lysozyme+, CD45RO+/-, HLA-DR+/-, CD4+/-, S100-/+,<br />
CD1a-, CD21-, CD35-, CD13, CD33, myeloperoxidase-, lack IGH and TCR gene rearrangements<br />
Langerhans Cell Histiocytosis: CD1a+, langerin+, S100+, vimentin+, CD68+, HLA-DR+, CD4-/+, CD30+,<br />
most B- and T-cell markers are negative, there are no consistent cytogenetic abnormalities<br />
Langerhans Cell Sarcoma: CD1a+, langerin+, S100+, vimentin+, CD68+, HLA-DR+, CD4-/+, CD30+, most<br />
B- and T-cell markers are negative, there are no consistent cytogenetic abnormalities<br />
Interdigitating Dendritic Cell Sarcoma: S100+, vimentin+, CD1a-, langerin-, CD45+/-, CD68+/-,<br />
lysozyme+/-, p53+/-, CD21-, CD23-, CD35-, CD34-, CD30-, myeloperoxidase-, most B and T-cell markers<br />
are negative, lack IGH and TCR gene rearrangements<br />
Follicular Dendritic Cell Sarcoma: Clusterin+, CD21+, CD35+, CD23+, KiM4p+, desmoplakin+, vimentin+,<br />
fascin+, EDGR+, HLA-DR+, CD1a-, myeloperoxidase-, lysozyme-, CD34-, CD30-, CD3-, CD79a-, lack IGH<br />
and TCR gene rearrangements<br />
Disseminated Juvenile Xanthogranuloma: vimentin+, CD14+, CD68+, CD163+, factor XIIIa+/-, fascin+/-,<br />
S100-/+, CD1a-, langerin-, lack IGH and TCR gene rearrangements
F. Clinical Prognostic Factors and Indices<br />
The specific histologic type <strong>of</strong> the lymphoid neoplasm, stage <strong>of</strong> disease, as well as the International<br />
Prognostic Index (IPI score) are the main factors used to determine treatment in adults. 13,21,28-33 The 5<br />
pretreatment characteristics that have been shown to be independently statistically significant are: age<br />
in years (≤60 versus >60); tumor stage I or II (localized) versus III or IV (advanced); number <strong>of</strong> extranodal<br />
sites <strong>of</strong> involvement (0 or 1 versus >1); patient’s performance status (0 or 1 versus 2 to 4); and serum LDH<br />
(normal versus abnormal). Based on the number <strong>of</strong> risk factors, patients can be assigned to 1 <strong>of</strong> 4 risks<br />
groups: low (0 or 1), low intermediate (2), high intermediate (3), or high (4 or 5). Patients stratified by the<br />
number <strong>of</strong> risk factors were found to have very different outcomes with regard to complete response<br />
(CR), relapse-free survival (RFS), and overall survival (OS). 13 Studies show that low-risk patients had an<br />
87% CR rate and an OS rate <strong>of</strong> 73% at 5 years compared to high-risk patients who had a 44% CR rate<br />
and a 26% 5-year overall survival rate. 13 A revised IPI (R-IPI) has been proposed for patients with diffuse<br />
large B-cell lymphoma who are treated with rituximab plus CHOP chemotherapy. 34 In pediatric cases,<br />
there is no equivalent <strong>of</strong> the IPI, and prognosis is based on stage and type <strong>of</strong> lymphoma. 16<br />
A separate prognostic index has become accepted for follicular lymphoma. The Follicular Lymphoma<br />
International Prognostic Index (FLIPI) appears to provide greater discrimination and stratification among<br />
patients with follicular lymphoma. 35 It evaluates 5 adverse prognostic risk factors including age (>60<br />
years versus ≤60 years), Ann Arbor stage (III to IV versus I to II), hemoglobin level (4 versus ≤4) and serum LDH level (above normal level versus normal or<br />
below). Patients are stratified into 3 risk groups: low risk (0-1 adverse factors), intermediate (2 adverse<br />
factors) and poor risk (≥3 adverse factors).<br />
Prognostic indices are also under development in other lymphoid neoplasms such as mantle cell<br />
lymphoma and T-cell lymphomas.<br />
Although not always provided to the pathologist by the physician submitting the specimen, certain<br />
specific clinical findings are known to be <strong>of</strong> prognostic value in all stages <strong>of</strong> NHL. In particular, systemic<br />
symptoms <strong>of</strong> fever (greater than 38°C), unexplained weight loss (more than 10% body weight) in the<br />
6 months before diagnosis, and drenching night sweats are used to define 2 categories for each stage<br />
<strong>of</strong> NHL: A (symptoms absent) and B (symptoms present). The presence <strong>of</strong> B symptoms is known to<br />
correlate with extent <strong>of</strong> disease (stage and tumor bulk), but symptoms also have been shown to have<br />
prognostic significance for cause-specific survival that is independent <strong>of</strong> stage. 6,28-33,36<br />
References<br />
1. Hussong JW, Arber DA, Bradley KT, et al. Protocol for the examination <strong>of</strong> specimens from patients<br />
with Hodgkin lymphoma. In: Reporting on Cancer Specimens: Case Summaries and Background<br />
Documentation. Northfield, IL: College <strong>of</strong> American Pathologists; 2009.<br />
2. Knowles D, ed. Neoplastic <strong>Hematopathology</strong>. Philadelphia, PA: Lippincott Williams and Wilkins;<br />
2001.<br />
3. Mills S, ed. Histology for Pathologists. Philadelphia, PA: Lippincott Williams and Wilkins; 2007.<br />
4. Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. World Health Organization Classification <strong>of</strong> Tumours:<br />
Pathology and Genetics <strong>of</strong> Tumours <strong>of</strong> Haematopoietic and Lymphoid Tissues. Lyon, France: IARC<br />
Press; 2001.<br />
5. Swerdlow S, Campo E, Harris N, Jaffe E, Pilero S, Stein H, Thiele J, Vardiman J, eds. WHO<br />
Classification <strong>of</strong> Tumours <strong>of</strong> Haematopoietic and Lymphoid Tissues. Geneva, Switzerland: WHO<br />
Press; 2008.<br />
6. Crump M, Gospodarowicz MK. Non-Hodgkin malignant lymphoma. In: Gospodarowicz MK, Henson<br />
DE, Hutter RVP, O’Sullivan B, Sobin LH, Wittekind C, eds. Prognostic Factors in Cancer. New York, NY:<br />
Wiley-Liss; 2001:689-703.<br />
7. Hsi E, Goldblum J, eds. <strong>Hematopathology</strong>. Philadelphia, PA: Churchill Livingstone Elsevier; 2007.
8. Craig F, Foon K. Flow cytometric immunophenotyping for hematologic neoplasms. Blood.<br />
2008;111(8):3941-3967.<br />
9. Jaffe E, Banks P, Nathwani B, et al. Recommendations for the reporting <strong>of</strong> lymphoid neoplasms: a<br />
report from the Association <strong>of</strong> Directors <strong>of</strong> Anatomic and Surgical Pathology. Mod Pathol.<br />
2004;17(1):131-135.<br />
10. Bagg A. Molecular diagnosis in lymphomas. Curr Oncol Rep. 2004;6(5):369-379.<br />
11. Carbone P, Kaplan H, Mussh<strong>of</strong>f K, et al. Report <strong>of</strong> the Committee on Hodgkin’s Disease Staging<br />
Classification. Cancer Res. 1971;31(11):1860-1861.<br />
12. Lister T, Crowther D, Sutcliffe S, et al. Report <strong>of</strong> a committee convened to discuss the evaluation<br />
and staging <strong>of</strong> patient’s with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630-<br />
1636.<br />
13. Lymphoid neoplasms. In: Edge SB, Byrd DR, Carducci MA, Compton CC, eds. AJCC Cancer<br />
Staging <strong>Manual</strong>. 7 th ed. New York, NY: Springer; 2009.<br />
14. Sobin LH, Gospodarowicz M, Wittekind Ch, eds. UICC TNM Classification <strong>of</strong> Malignant Tumours. 7th<br />
ed. New York, NY: Wiley-Liss; 2009.<br />
15. Durie B, Salmon S. A clinical staging system for multiple myeloma: correlation <strong>of</strong> measured<br />
myeloma mass with presenting clinical features, response to treatment, and survival. Cancer.<br />
1975;36(3):842-854.<br />
16. Cairo, et al. Non-Hodgkin’s lymphoma in children. In: Kufe P, Weishelbuam R, et al, eds. Cancer<br />
Medicine. 7 th ed. London: BC Decker; 2006:1962-1975.<br />
17. Armitage J. Staging non-Hodgkin lymphoma. CA Cancer J Clin. 2005;55(6):368-376.<br />
18. Ansell S, Armitage J. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc.<br />
2005;80(8):1087-1097.<br />
19. Kwee T, Kwee R, Nievelstein R. Imaging in staging malignant lymphoma: a systematic review. Blood.<br />
2008;111(2):504-516.<br />
20. Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification <strong>of</strong> mycosis<br />
fungoides and Sézary syndrome: a proposal <strong>of</strong> the International Society for Cutaneous Lymphomas<br />
(ISCL) and the cutaneous lymphoma task force <strong>of</strong> the European Organization <strong>of</strong> Research and<br />
Treatment <strong>of</strong> Cancer (EORTC). Blood. 2007;110(6):1708-1709.<br />
21. Zelenetz A, Hoppe R. NCCN: non-Hodgkin’s lymphoma. Cancer Control. 2001:8(6 suppl 2):102-113.<br />
22. Arber DA. Molecular approach to non-Hodgkin’s lymphoma. J Mol Diagn. 2000:2(4);178-190.<br />
23. Bagg A. Role <strong>of</strong> molecular studies in the classification <strong>of</strong> lymphoma. Expert Rev Mol Diagn.<br />
2004;4(1):83-97.<br />
24. Sen F, Vega F, Medeiros LJ. Molecular genetic methods in the diagnosis <strong>of</strong> hematologic neoplasms.<br />
Semin Diagn Pathol. 2002;19(2):72-93.<br />
25. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification <strong>of</strong> lymphoid<br />
neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392.<br />
26. Chan JK, Banks PM, Cleary ML, et al. A revised European-American classification <strong>of</strong> lymphoid<br />
neoplasms: a proposal from the International Lymphoma Study Group: a summary version. Am J<br />
Clin Pathol. 1995;103(5):543-560.<br />
27. Nguyen D, Diamond L, Braylan R. Flow Cytometry in <strong>Hematopathology</strong>: A Visual Approach to Data<br />
Analysis and Interpretation. Totowa, NJ: Humana Press; 2003.<br />
28. A predictive model for aggressive non-Hodgkin’s lymphoma: The International Non-Hodgkin’s<br />
Lymphoma Prognostic Factors Project. N Engl J Med. 1993;329(14):987-994.<br />
29. Shipp M. Prognostic factors in aggressive non-Hodgkin lymphoma. Blood. 1994;83(5):1165-1173.<br />
30. Hoskins PJ, Ng V, Spinelli JJ, et al. Prognostic variables in patients with diffuse large-cell lymphoma<br />
treated with MACOP-B. J Clin Oncol. 1991;9(2):220-226.<br />
31. Cowan RA, Jones M, Harris M, et al. Prognostic factors in high and intermediate grade non-Hodgkin<br />
lymphoma. Br J Cancer. 1989;59(2):276-282.<br />
32. Gospodarowicz MK, Bush RS, Brown TC, et al. Prognostic factors in nodular lymphomas: a<br />
multivariate analysis based on the Princess Margaret Hospital experience. Int J Radiat Oncol Biol<br />
Phys. 1984;10(4):489-497.
33. Osterman B, Cavallin-Stahl E, Hagberg H, et al. High-grade non-Hodgkin lymphoma<br />
stage I: a retrospective study <strong>of</strong> treatment, outcome, and prognostic factors in 213<br />
patients. Acta Oncol.<br />
1996;35(2):171-177.<br />
34. Sehn L, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is<br />
a better predictor <strong>of</strong> outcome than the standard IPI for patients with diffuse large B-cell<br />
lymphoma treated with R-CHOP. Blood. 2007;109(5):1857-1861.<br />
35. Solal-Celigny P, Roy P, Colombat P, et al. Follicular Lymphoma International Prognostic<br />
Index.<br />
Blood. 2004;104(5):1258-1265.<br />
36. Velasquez WS, Jagannath S, Tucker SL, et al. Risk classification as the basis for clinical<br />
staging <strong>of</strong> diffuse large-cell lymphoma derived from 10-year survival data. Blood.<br />
1989;74(2):551-557.<br />
18
Protocol for the Examination <strong>of</strong> Specimens from Patients<br />
with Hodgkin Lymphoma<br />
Protocol applies to Hodgkin lymphoma involving any site. #<br />
Based on AJCC/UICC TNM, 7 th Edition<br />
Protocol web posting date: October 2009<br />
Procedures<br />
Biopsy<br />
Resection <strong>of</strong> Lymph Node(s) or Other Organ(s)<br />
Authors<br />
Jerry W. Hussong, MD, DDS, FCAP*<br />
Cedars-Sinai Medical Center, Los Angeles, California<br />
Daniel A. Arber, MD<br />
Stanford University School <strong>of</strong> Medicine, Stanford, California<br />
Kyle T. Bradley MD, MS, FCAP<br />
Emory University Hospital, Atlanta, Georgia<br />
Michael S. Brown, MD, FCAP<br />
Yellowstone Pathology Institute Inc, Billings, Montana<br />
Chung-Che Chang, MD, PhD, FCAP<br />
The Methodist Hospital, Houston, Texas<br />
Monica E. de Baca, MD, FCAP<br />
Physicians Laboratory Ltd, Sioux Falls, South Dakota<br />
David W. Ellis, MBBS, FRCPA<br />
Flinders Medical Centre, Bedford Park, South Australia<br />
Kathryn Foucar, MD, FCAP<br />
University <strong>of</strong> New Mexico, Albuquerque, New Mexico<br />
Eric D. Hsi, MD, FCAP<br />
Cleveland Clinic Foundation, Cleveland, Ohio<br />
Elaine S. Jaffe, MD<br />
National Cancer Institute, Bethesda, Maryland<br />
Michael Lill, MB, BS, FRACP, FRCPA<br />
Cedars-Sinai Medical Center, Los Angeles, California<br />
Stephen P. McClure, MD<br />
Presbyterian Pathology Group, Charlotte, North Carolina<br />
L. Jeffrey Medeiros, MD, FCAP<br />
MD Anderson Cancer Center, Houston, Texas<br />
Sherrie L. Perkins, MD, PhD, FCAP<br />
University <strong>of</strong> Utah Health Sciences Center, Salt Lake City, Utah<br />
For the Members <strong>of</strong> the Cancer Committee, College <strong>of</strong> American Pathologists<br />
*denotes the primary and senior author. All other contributing authors are listed alphabetically.<br />
© 2009 College <strong>of</strong> American Pathologists (CAP). All rights reserved.<br />
A. Specimen<br />
Any number <strong>of</strong> specimen types may be submitted in the evaluation <strong>of</strong> Hodgkin lymphoma.<br />
Lymph nodes, mediastinal masses, bone marrow, spleen, lung, and liver are among the most<br />
common. Specimens submitted with a suspected diagnosis <strong>of</strong> Hodgkin lymphoma require<br />
special handling in order to optimize the diagnosis. Often, lymph node specimens are submitted<br />
19
where the differential diagnosis includes both Hodgkin and non-Hodgkin lymphomas, and, if<br />
possible, tissue should be obtained for possible molecular and other ancillary studies, which are<br />
<strong>of</strong>ten necessary for the diagnosis <strong>of</strong> non-Hodgkin lymphomas. 1,2 Most flow cytometry,<br />
molecular, and cytogenetic studies will not aid in the diagnosis <strong>of</strong> Hodgkin lymphoma.<br />
Immunophenotyping by immunohistochemical staining is necessary in the initial diagnosis <strong>of</strong><br />
nearly all cases <strong>of</strong> Hodgkin lymphoma. Because <strong>of</strong> this, well-fixed sections are <strong>of</strong> paramount<br />
importance. The guidelines detailed below are suggested for specimen handling in cases <strong>of</strong><br />
suspected Hodgkin lymphoma.<br />
Tissue should be received fresh. Unsectioned lymph nodes should not be immersed<br />
in fixative, and care should be taken to make thin (2 mm) slices perpendicular to the long<br />
axis <strong>of</strong> the node to ensure optimal penetration <strong>of</strong> fixative.<br />
The fresh specimen size, color, and consistency should be recorded, as should the<br />
presence or absence <strong>of</strong> any visible nodularity, hemorrhage, or necrosis.<br />
Touch imprints may be made from the freshly cut surface, and the imprints fixed in alcohol<br />
or air dried. Unstained air-dried imprints can be used for fluorescence in situ hybridization<br />
(FISH) or other studies if necessary.<br />
For microbiology studies: submit a fresh portion <strong>of</strong> the lymph node (or other specimen type)<br />
sterilely in appropriate medium.<br />
Flow cytometry immunophenotyping is not routinely used in the diagnosis <strong>of</strong> Hodgkin<br />
lymphoma, but if the differential diagnosis includes non-Hodgkin lymphoma, a fresh portion<br />
<strong>of</strong> the specimen should be submitted in appropriate transport medium such as RPMI.<br />
Fixation (record fixative[s] used for individual slices <strong>of</strong> the specimen):<br />
o Estimated time from excision to fixation should be noted, if possible, as this may impact<br />
preservation or recovery <strong>of</strong> certain analytes such as RNA and phosphoproteins in fixed<br />
tissues.<br />
o Zinc formalin or B+ produces superior cytologic detail but is not suitable for DNA<br />
extraction and may impair some immunostains (eg, CD30). B+ also has the additional<br />
limitation <strong>of</strong> requiring proper hazardous materials disposal.<br />
o Formalin fixation is preferable when the tissue sample is limited, as it is most suitable for<br />
immunohistochemistry as well as many other ancillary tests such as molecular/genetic<br />
studies and in-situ hybridization.<br />
o Over-fixation (ie, more than 24 hours in formalin, more than 4 hours in zinc formalin or<br />
B+) should be avoided for optimal immunophenotypic reactivity.<br />
B. Tumor Site<br />
Hodgkin lymphomas are nearly always nodal based with cervical lymph nodes more commonly<br />
involved. It can also frequently be seen involving mediastinal, axillary, and paraaortic lymph<br />
nodes. Extranodal Hodgkin lymphoma can rarely be seen. The anatomic distribution <strong>of</strong><br />
Hodgkin lymphoma, however, varies depending on the histologic type. 3<br />
C. Histologic Type<br />
This protocol recommends assigning histologic type based on the World Health Organization<br />
(WHO) classification <strong>of</strong> lymphoid neoplasms. 4 It was originally published in 2001 and more<br />
recently revised and updated in 2008. 4,5 This classification encompasses both Hodgkin and<br />
non-Hodgkin lymphomas and allows distinction <strong>of</strong> individual lymphoid neoplasms based upon<br />
morphologic, immunophenotypic, cytogenetic, and clinical features. While histologic<br />
examination typically is thought to be the gold standard, the majority <strong>of</strong> Hodgkin lymphomas will<br />
require immunohistochemical staining, especially at the time <strong>of</strong> initial diagnoses. 4-9 In addition,<br />
while Hodgkin lymphomas are currently divided into nodular lymphocyte predominant Hodgkin<br />
lymphoma and classical Hodgkin lymphomas (including nodular sclerosis, mixed cellularity,<br />
20
lymphocyte-rich, and lymphocyte-depleted subtypes), it should be recognized that classical<br />
Hodgkin lymphomas may not represent a single disease. In addition, there is overlap between<br />
some cases <strong>of</strong> Hodgkin lymphoma and non-Hodgkin lymphoma, particularly diffuse large B-cell<br />
lymphomas (so-called gray zone lymphomas). 4,10<br />
D. Pathologic Extent <strong>of</strong> Tumor (Stage)<br />
The TNM classification is not used for staging Hodgkin lymphomas because the site <strong>of</strong> origin <strong>of</strong><br />
the tumor is <strong>of</strong>ten unclear and there is no way to differentiate among T, N, and M. The Cotswold<br />
revision <strong>of</strong> the Ann Arbor staging classification is used for Hodgkin lymphoma. 11,12 It was<br />
originally published over 30 years ago.<br />
Pathologic staging depends on the biopsy <strong>of</strong> multiple lymph nodes on both sides <strong>of</strong> the<br />
diaphragm, splenectomy, wedge liver biopsy, and bone marrow biopsy to assess distribution <strong>of</strong><br />
disease.<br />
Currently, staging for Hodgkin lymphoma is more commonly clinical than pathologic. Clinical<br />
staging generally involves a combination <strong>of</strong> clinical, radiologic, and surgical data. Physical<br />
examination, laboratory tests, imaging studies (eg, computed tomography [CT] scans, magnetic<br />
resonance imaging [MRI] studies, and positron emission tomography [PET]), biopsy (to<br />
determine diagnosis, histologic type, and extent <strong>of</strong> disease), and bone marrow examination are<br />
<strong>of</strong>ten required. Correct diagnosis and staging are the key factors in providing appropriate<br />
treatment. 13-15<br />
Cotswold Revision <strong>of</strong> the Ann Arbor Staging Classification <strong>of</strong> Hodgkin<br />
Lymphomas 13,14<br />
Stage I Involvement <strong>of</strong> a single lymph node region (I), or lymphoid structure (eg, spleen,<br />
thymus, Waldeyer’s ring). #<br />
Stage II Involvement <strong>of</strong> 2 or more lymph node regions on the same side <strong>of</strong> the diaphragm<br />
(II) (the mediastinum is considered a single site). ##<br />
Stage III Involvement <strong>of</strong> lymph node regions on both sides <strong>of</strong> the diaphragm (III) which<br />
may be accompanied by extralymphatic extension in association with lymph node<br />
involvement (IIIE) or splenic involvement (IIIS).<br />
Stage IV Involvement <strong>of</strong> extranodal site(s) beyond those designated E.<br />
# Multifocal involvement <strong>of</strong> a single extralymphatic organ is classified as stage IE and not stage<br />
IV.<br />
## The number <strong>of</strong> lymph node regions involved may be indicated by a subscript: eg, II 3 .<br />
E designates involvement <strong>of</strong> a single extranodal site or contiguous or proximal known nodal site<br />
<strong>of</strong> disease.<br />
E. Immunophenotyping<br />
Immunophenotyping by flow cytometry and molecular testing by polymerase chain reaction<br />
(PCR) are currently not typically used or are not necessary for the diagnosis <strong>of</strong> Hodgkin<br />
lymphoma. Immunophenotyping using immunohistochemistry is necessary for the initial<br />
diagnosis <strong>of</strong> nearly all cases <strong>of</strong> Hodgkin lymphoma. It requires well-fixed tissue sections for<br />
optimal immunohistochemical staining and interpretation.<br />
21
Immunophenotypes 1,4-8<br />
The following is to be used as a guideline for the more common immunophenotype for each<br />
subtype <strong>of</strong> Hodgkin lymphoma. It is however, not entirely comprehensive and individual cases<br />
may vary somewhat in their immunophenotypic pr<strong>of</strong>ile.<br />
Nodular lymphocyte predominant Hodgkin lymphoma: Lymphocyte predominant cells (LP cells;<br />
previously called L&H cells) are CD20+, CD79a+, PAX5+, CD45+, BCL6+, OCT-2+, BOB.1+,<br />
EMA +/-, CD15-, CD30-, CD43-, EBER-.<br />
Nodular sclerosis classical Hodgkin lymphoma: Classical Hodgkin/Reed-Sternberg cells are<br />
CD30+, CD15+/-, CD45-, PAX5+/-, CD20-/+, CD79a-/+, EBER-/+, OCT-2-/+,<br />
BOB.1-/+, EMA-<br />
Mixed cellularity classical Hodgkin lymphoma: Classical Hodgkin/Reed-Sternberg cells are<br />
CD30+, CD15+/-, CD45-, PAX5+/-, CD20-/+, CD79a-/+, EBER+/-, OCT-2-/+,<br />
BOB.1-/+, EMA-<br />
Lymphocyte-rich classical Hodgkin lymphoma: Classical Hodgkin/Reed-Sternberg cells are<br />
CD30+, CD15+/-, CD45-, PAX5+/-, CD20-/+, CD79a-/+, EBER-/+, OCT-2-/+,<br />
BOB.1-/+, EMA-<br />
Lymphocyte-depleted classical Hodgkin lymphoma: Classical Hodgkin/Reed-Sternberg cells are<br />
CD30+, CD15+/-, CD45-, PAX5+/-, CD20-/+, CD79a-/+, EBER+/-, OCT-2-/+, BOB.1-/+, EMA-<br />
F. Clinical Prognostic Factors and Indices<br />
The International Prognostic Score (IPS) was developed for Hodgkin lymphoma to predict<br />
outcome based on the following adverse factors: serum albumin
3. Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-rich classical<br />
Hodgkin’s lymphoma: clinical presentation and treatment outcome in 100 patients treated<br />
within German Hodgkin’s Study Group trials. J Clin Oncol. 2005; 23(24):5739-5745.<br />
4. Swerdlow S, Campo E, Harris N, Jaffe E, Pilero S, Stein H, Thiele J, Vardiman J, eds.<br />
WHO Classification <strong>of</strong> Tumours <strong>of</strong> Haematopoietic and Lymphoid Tissues. Geneva,<br />
Switzerland: WHO Press; 2008.<br />
5. Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Pathology and Genetics <strong>of</strong> Tumours <strong>of</strong><br />
Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. World Health<br />
Organization Classification <strong>of</strong> Tumours, Vol. 3.<br />
6. Hsi E, Goldblum J, eds. <strong>Hematopathology</strong>. Philadelphia, PA: Churchill Livingstone<br />
Elsevier; 2007.<br />
7. Zukerberg L, Collins AB, Ferry JA, Harris NL. Coexpression <strong>of</strong> CD15 and CD20 by Reed-<br />
Sternberg cells in Hodgkin’s disease. Am J Pathol. 1991; 139(3):475-483.<br />
8. Jaffe E, Banks P, Nathwani B, et al. Recommendations for the reporting <strong>of</strong> lymphoid<br />
neoplasms: a report from the Association <strong>of</strong> Directors <strong>of</strong> Anatomic and Surgical Pathology.<br />
Mod Pathol. 2004; 17(1):131-135.<br />
9. Stein H, Marafioti T, Foss H, et al. Down-regulation <strong>of</strong> BOB.1/OBF.1 and Oct2 in classical<br />
Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with<br />
immunoglobulin transcription. Blood. 2001; 97(2):496-501.<br />
10. Mani H, Jaffe E. Hodgkin lymphoma: an update on its biology with new insights into<br />
classification. Clin Lymphoma Myeloma. 2009; 9(3):206-216.<br />
11. Carbone P, Kaplan H, Mussh<strong>of</strong>f K, et al. Report <strong>of</strong> the Committee on Hodgkin’s Disease<br />
Staging Classification. Cancer Res. 1971; 31(11):1860-1861.<br />
12. Lister T, Crowther D, Sutcliffe S, et al. Report <strong>of</strong> a committee convened to discuss the<br />
evaluation and staging <strong>of</strong> patient’s with Hodgkin’s disease: Cotswolds meeting. J Clin<br />
Oncol. 1989;7(11):1630-1636.<br />
13. Lymphoid neoplasms. In: Edge SB, Byrd DR, Carducci MA, Compton CC, eds. AJCC<br />
Cancer Staging <strong>Manual</strong>. 7 th ed. New York, NY: Springer; 2009.<br />
14. Sobin LH, Gospodarowicz M, Wittekind Ch, eds. UICC TNM Classification <strong>of</strong> Malignant<br />
Tumours. 7th ed. New York, NY: Wiley-Liss; in press.<br />
15. Kwee T, Kwee R, Nievelstein R. Imaging in staging malignant lymphoma: a systematic<br />
review. Blood. 2008; 111(2):504-516.<br />
16. Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease: International<br />
Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;<br />
339(21):1506-1514.<br />
17. Allemani C, Sant M, De Angelis R, et al. Hodgkin disease survival in Europe and the U.S.:<br />
prognostic significance <strong>of</strong> morphologic groups. Cancer. 2006; 107(2):352-360.<br />
18. Vassilakopoulos T, Angelopoulou M, Siakantaris M, et al. Prognostic factors in advanced<br />
stage Hodgkin’s lymphoma: the significance <strong>of</strong> the number <strong>of</strong> involved anatomic sites. Eur<br />
J Haematol. 2001; 67(5-6):279-288.<br />
19. Sup J, Alemany C, Pohlman B, et al. Expression <strong>of</strong> bcl-2 in classical Hodgkin’s lymphoma:<br />
an independent predictor <strong>of</strong> poor outcome. J Clin Oncol. 2005;23(16):3773-3779.<br />
20. Rautert R, Schinkothe T, Franklin J, et al. Elevated pretreatment interleukin-10 serum level<br />
is an International Prognostic Score (IPS)-independent risk factor for early treatment failure<br />
in advanced stage Hodgkin lymphoma. Leuk Lymphoma. 2008; 49(11):2091-2098.<br />
21. Rassidakis G, Medeiros LJ, Vassilakopoulos T, et al. Bcl-2 expression in Hodgkin and<br />
Reed-Sternberg cells <strong>of</strong> classical Hodgkin lymphoma predicts a poorer prognosis in<br />
patients treated with AVBD or equivalent regimens. Blood. 2002; 100(12):3935-3941.<br />
23
Immunohistochemistry
Immunostains/In-Situ Hybridization Laboratory and Ordering<br />
Information<br />
Immunostain Turnaround Time<br />
IHC Routine runs: All orders received before 10:00am should be sent out the same day by<br />
5:30pm (i.e. Monday through Friday) provided the laboratory has the block (or slides). There<br />
are a few stains (e.g. EBER, SV40, TdT, and antibody reactions on frozens) that require longer<br />
incubations and these may be delayed by one day. Orders received after 10:00am will be out<br />
the following morning by the 8:30am courier.<br />
The Immunohistochemistry Laboratory is located at the CLB and personnel can be reached at<br />
647-7663.<br />
A list <strong>of</strong> the antibodies we use most frequently and a complete list with codes are included in the<br />
manual.<br />
ORDERING OF IMMUNOSTAIN PANELS<br />
It is strongly recommended that the Audix voicemail (412-803-3273) is utilized when ordering<br />
stains in order to facilitate delivery to <strong>Hematopathology</strong>.<br />
If ordering after hours, the stain panels must now be ordered as Stain/Process Group Protocols<br />
(see separate list).<br />
(Unlike a Histology Protocol, a Stain/Process Group Protocol will not write over stains that have<br />
been previously ordered on the same part)<br />
To order one <strong>of</strong> the protocols:<br />
1. If you are ordering the panel using the “Accession Entry/Edit” or “Histology Entry/Edit”<br />
activities:<br />
• Go to the Histology tab.<br />
• Select the part (and/or block) on which you wish to order the panel.<br />
• Then click on the “Run Stain/Process Group…” button.<br />
• Enter the abbreviation for the Protocol in the “Select Protocol” field on the “Run<br />
Stain/Process Group Protocol” pop up window.<br />
• Hit tab (on your keyboard). Then hit Enter (on your keyboard) or click the OK button in the<br />
“Run Stain/Process Group Protocol” pop up window.
2. If you are ordering the panel using the “Stain/Process and Block Edit” activity:<br />
• Select the part (and/or block) on which you wish to order the panel.<br />
• Click on the “Add Stain/Process…” button.<br />
• Clinical on the “Run Stain/Process Group…” button.<br />
• Enter the abbreviation for the Protocol in the “Select Protocol” field on the “Run<br />
Stain/Process Group Protocol” pop up window.<br />
• Hit tab (on your keyboard). Then hit Enter (on your keyboard) or click the OK button in the<br />
“Run Stain/Process Group Protocol” pop up window.<br />
In the comment field enter” Please deliver to <strong>Hematopathology</strong>” – otherwise the slides may end<br />
up in your mailbox!!<br />
If you have any questions, please contact AP User Support via e-mail or at 647-9170<br />
ORDERING EXPERIMENTAL STAINS (ANTIBODIES NOT YET IN COMPUTER)<br />
If being done in the routine immunohistology laboratory, order under ABNKNC. Specify desired<br />
stain in the comment <strong>of</strong> ABNKNC.<br />
If being done in the in situ laboratory order 2 blanks (IBNKNC) and write in comment to sent to<br />
insitu laboratory for_________.<br />
Reporting <strong>of</strong> Immunostains/in-situ hybridization<br />
The template should always be utilized and follows the general microscopic description.
Stains Frequently Used In <strong>Hematopathology</strong>: Codes and Reactivities<br />
Here’s a list <strong>of</strong> markers that are used commonly on the Lymph Node Service and their secret<br />
Client Server code-names. See also the <strong>Hematopathology</strong> worksheet since its best to order via<br />
panels.<br />
Antigen /<br />
Marker<br />
C/S Codename<br />
Cell-type/s<br />
CD1a ACD1 Thymocytes, Langerhans Cells<br />
CD2 ACD2 T-cells, NK-cells<br />
CD3 ACD3 T-cells, NK-cells<br />
CD4 ACD4 T-cells, Macrophage<br />
CD5 ACD5 T-cells, SLL/CLL & Mantle cell<br />
lymphoma, B-cell subset<br />
CD7 ACD7 T-cells, NK-cells<br />
CD8 ACD8 T-cells, NK-cells<br />
CD10 ACD10 Lymphoblasts, Follicular center<br />
(CALLA)<br />
cells, Granulocytes, Some<br />
epithelial cells/neoplasms,<br />
Follicular T-cells<br />
CXCL-13 ACXCL13 Follicular T helper cells<br />
PD-1 APDT Follicular T helper cells<br />
CD15 (Leu- ALEUM1 R-S cells, CMV-infected cells,<br />
M1)<br />
Some carcinomas<br />
CD20 (L26) AL26 B-cells<br />
CD21 ACD21 Follicular dendritic cells, Some B-<br />
cells<br />
CD22 ACD22 B-cells<br />
CD23 ACD23 Follicular dendritic cells,<br />
SLL/CLL, Normal mantle cells<br />
CD30 (Ki-<br />
1/Ber-H2)<br />
ACD30<br />
R-S cells, Activated T & B-cells,<br />
Some lymphomas, Embryonal<br />
carcinoma<br />
CD31 ACD31 Endothelium<br />
CD33 ACD33 Myeloid<br />
CD34 ACD34 Blasts, Endothelium, Various<br />
mesenchymal neoplasms<br />
CD43 ACD43 T-cells, Myeloid cells, B-cell<br />
subset, SLL/CLL, Mantle cell<br />
lymphoma, Other B-cell<br />
lymphomas, Not most follicular<br />
lymphomas<br />
CD45 (LCA) ALCA All leukocytes<br />
CD45RA<br />
(4KB5)<br />
ACD45R B-cells, Plasmacytoid dendulic<br />
cells<br />
CD45RO AUCHL1 T-cells, Macrophages<br />
(UCHL-1)<br />
CD56 ACD56 NK-cells, “NK-like” T-cells; Some<br />
malignant myeloblasts,<br />
lymphoblasts & plasma cells<br />
(multiple myeloma)<br />
Comment
CD57 (Leu<br />
7)<br />
ALEU7 NK-cells, Neural tissues, T-cell<br />
subset in follicles<br />
CD61 ACD61B Megakaryocytes<br />
CD68 ACD68 Monocyte/Macrophage, Many<br />
Melanomas!<br />
CD79a ACD79 B-cells<br />
CD99 AEWING Blasts, Ewing Sarcoma<br />
CD138 ACD138 Plasma cells, R-S cells, Some<br />
epithelial cells<br />
Alk-1 AALK Anaplastic large cell lymphoma<br />
(systemic)<br />
TIA-1 ATIA T-cells & NK-cells with cytolytic<br />
granules<br />
Granzyme B AGRANZ Cytotoxic T-cells and NK cells<br />
Clusterin ACLUST Positive staining in anaplastic<br />
large cell lymphomas<br />
Bcl-2 ABCL2 Many normal cells but NOT<br />
NORMAL germinal center cells;<br />
Majority <strong>of</strong> low grade follicular<br />
lymphomas, fewer intermediate<br />
grade ones; Many other<br />
lymphomas<br />
Cyclin-D1<br />
(bcl-1)<br />
ACYCLD<br />
Mantle cell lymphoma, Some<br />
multiple myelomas, Epithelial<br />
cell/neoplasms<br />
Bcl-6 ABCL6 Germinal center B-cells (follicular<br />
center cells); Many diffuse large<br />
B-cell lymphomas<br />
Ki-67 (MIB-<br />
1)<br />
TdT(Termina<br />
l-<br />
deoxynucleo<br />
tidyl<br />
Transferase)<br />
MPO<br />
(Myeloperoxi<br />
dase)<br />
AKI67<br />
ATDT<br />
AMPO<br />
Proliferation marker (G1/S/G2/M<br />
phases)<br />
Lymphoblasts; Sometimes<br />
myeloblasts (
Beta-F1 ABF1 Beta-F1 T-cell receptor chain on<br />
T-cells<br />
Glycophorin- AGLYP RBC’s and their precursors<br />
A<br />
PAX5 APAX5 B-cells (nuclear stain)<br />
EBV EBER-<br />
ISH<br />
IEBER Epstein-Barr Virus Encoded<br />
mRNA (in situ hybridization)<br />
Kappa IRKAP Ig light chain kappa mRNA (in<br />
mRNA-ISH<br />
situ hybridization)<br />
p27 AP27 Pos in indolent B-cell lymphomas<br />
but not MCL<br />
Lambda IRLAM Ig light chain lambda mRNA (in<br />
mRNA-ISH<br />
situ hybridization)<br />
Kappa/Lamb IKPLM Kappa is black /Lambda is redorange<br />
da double<br />
(antibody stain performed<br />
stain<br />
in in-situ hybridization lab)<br />
Bartonella CABART Bartonella henselae<br />
henselae<br />
stain<br />
Order under Run<br />
Stain/Process Group<br />
Order under Run<br />
Stain/Process Group<br />
Order under Run<br />
Stain/Process Group<br />
Order under Run<br />
Stain/Process Group
Complete Immunohistochemical Stain Abbreviations/Codes<br />
See online library for detailed information re: clones, etc<br />
http://aplis.upmc.edu/aplis/resources/index.cfm<br />
Antibody<br />
Actin - Smooth muscle (SMA)<br />
Actin All Muscle<br />
Adenovirus<br />
Adhalin<br />
Adrenocorticotropic Hormone<br />
AE1<br />
AE1/3<br />
AE3<br />
Albumin<br />
Alpha 1 Antichymotrypsin<br />
Alpha 1 Antitrypsin<br />
Alpha-Fetoprotein<br />
Alzheimer beta Amyloid<br />
Amyloid A<br />
Amyloid P<br />
Anaplastic Lymphoma Kinase<br />
androgen receptor<br />
Annexin-1<br />
APP<br />
B72.3/TAG Tumor Glycoprotein<br />
Bartonella Henselae<br />
Bcl-2 Oncoprotein<br />
BCL6<br />
BerEP4<br />
Beta Catenin<br />
BetaF1 T-cell Receptor<br />
BHCG<br />
BK Virus<br />
BOB1<br />
BRACHYURY<br />
Test Code<br />
AACTIN<br />
AMA<br />
Adenovirus<br />
Adhalin<br />
ACTH<br />
AE1<br />
AE1/3<br />
AE3<br />
Albumin<br />
AACT<br />
AAT<br />
AFP<br />
A4G8<br />
Amyloid A<br />
Amyloid P<br />
AALK1<br />
AAR<br />
Annexin-1<br />
APP<br />
ATAG<br />
Bhenselae<br />
ABCL2<br />
ABCL6<br />
ABEREP4<br />
ABCATN<br />
ABF1<br />
ABHCG<br />
ABKV<br />
ABOB1<br />
BRACHYURY
BRAF<br />
BRAF-GI<br />
C1Q Complement<br />
AC1Q<br />
C4d<br />
AC4D<br />
C4d Frozen<br />
AC4Df<br />
C5B-9 Membrane Complex<br />
APMAC<br />
CA125<br />
ACA125<br />
CA19.9<br />
YCA199<br />
CA9<br />
ACA9<br />
Calcitonin<br />
ACALCI<br />
Caldesmon<br />
ACALDES<br />
Calponin<br />
ACALP<br />
Calretinin<br />
ACALRET<br />
CAM 5.2 CAM 5.2<br />
Caveolin-1<br />
ACAVEOLIN-1<br />
CD10 (CALLA)<br />
ACD10<br />
CD117 C-KIT<br />
CD117<br />
CD138<br />
ACD138<br />
CD15<br />
ACD15<br />
CD1a<br />
ACD1A<br />
CD2<br />
ACD2<br />
CD20 - L26<br />
CD20<br />
CD21<br />
CD21<br />
CD22<br />
CD22<br />
CD23<br />
CD23<br />
CD25 INTERLEUKIN2<br />
ACD25<br />
CD3<br />
CD3<br />
CD30<br />
CD30<br />
CD31<br />
ACD31<br />
CD33<br />
CD33<br />
CD34<br />
CD34<br />
CD4<br />
CD4<br />
CD4 Frozen<br />
CD4f<br />
CD43<br />
CD43<br />
CD45RA<br />
CD45RA<br />
CD45RO - OPD4<br />
CD4
CD5<br />
CD5<br />
CD56<br />
CD56<br />
CD57<br />
CD57<br />
CD61<br />
CD61<br />
CD68<br />
CD68<br />
CD7<br />
CD7<br />
CD79a<br />
CD79a<br />
CD8<br />
CD8<br />
CD99 - Ewing<br />
CD99<br />
CDX2<br />
CDX2<br />
CEA<br />
ACEA<br />
CEA-M<br />
CEAM<br />
Chromogranin<br />
ACHROMO<br />
CITED-1<br />
ACITED-1<br />
CKIT - CD117<br />
ACKIT<br />
CLUSTERIN<br />
ACLUST<br />
COLLAGEN IV<br />
ACOL4<br />
Connexin 43<br />
COX2 - Cyclooxygenase-2<br />
ACOX2<br />
CRP<br />
CRP<br />
CXCL13 CXCL 13<br />
Cyclin D1 - BCL-1<br />
ACYCLD1<br />
Cytokeratin - Pan Keratin<br />
APANKLAM<br />
Cytokeratin 19<br />
ACK19<br />
Cytokeratin 20 - CK20<br />
ACK20<br />
Cytokeratin 5<br />
CK5<br />
Cytokeratin 5/6 - CK5/6<br />
ACK5/6<br />
Cytokeratin 7 - CK7<br />
ACK7<br />
Cytokeratin AE1/AE3 - CK AE1/3<br />
AE1/3<br />
Cytomegalovirus<br />
ACMV<br />
D2-40 AD240<br />
Desmin<br />
ADESMIN<br />
DOG1<br />
DOG1<br />
Dysferlin<br />
ADYSF<br />
Dystrophin1<br />
DYS1
Dystrophin2<br />
DYS2<br />
E-Cadherin<br />
ECAD<br />
EGFR<br />
AEGFR<br />
EMA<br />
AEMA<br />
Epstein Barr virus - EBV<br />
AEBV<br />
ER<br />
AER<br />
ERG<br />
ERG<br />
Ewing sarcoma - CD99<br />
AEWING<br />
Factor 8 - vonWildebrand<br />
AVWF<br />
Factor XIIIa<br />
AXIII<br />
Fibrinogen<br />
FIBRINOGEN<br />
FLI1<br />
FLI-1<br />
Fmac<br />
AFMAC<br />
Follicle Stim. Hormone<br />
FSH<br />
FUMARATE HYDRATASE<br />
FUM-HYD<br />
Galectin-3<br />
Galectin-3<br />
Gastrin<br />
Gastrin<br />
GCDFP15<br />
AGCDFP<br />
Glial Fibrillary Acidic Protein - GFAP AGFAP<br />
Glucagon<br />
GLUC<br />
Glut-1<br />
AGLUT1<br />
Glutamine Synthetase<br />
AGLUTSYNTH<br />
Glycophorin A<br />
AGLYP<br />
Glypican-3<br />
GPC-3<br />
Granzyme B<br />
AGRANZB<br />
Growth Hormone<br />
AGH<br />
H. pylori HPYL<br />
HBME-1<br />
AHBME<br />
Hemoglobin<br />
AHEMO<br />
Hepatitis B Core<br />
AHBCOR<br />
Hepatitis B Surface<br />
AHBS<br />
Hepatitis C<br />
AHEPC<br />
HepPar 1 Hepatocytes<br />
AHEPAR<br />
Her-2/neu c-erb<br />
ANEU<br />
Herpes Simplex Virus I & II<br />
AHSV12
HHV8<br />
HMB45 Melanoma<br />
HPV Papilloma Virus<br />
HSP70<br />
HSV1<br />
HSV2<br />
IDH1<br />
IgA<br />
IgD<br />
IgG<br />
IgG4<br />
IgM<br />
Inhibin Alpha<br />
Insulin<br />
Interleukin2 - CD25<br />
J chain <strong>of</strong> IgA+IgM<br />
Kappa amyloid<br />
Kappa Light Chain<br />
KI67 Proliferating Cells<br />
KI67 Proliferating Cells<br />
LAM/PANK<br />
Lambda BM<br />
Lambda Light Chain<br />
Laminin<br />
LAMY<br />
Langerin<br />
LCA, CD45 Monoclonal<br />
Leu M1 - CD15<br />
LN3 HLADR<br />
Lutenizing Hormone<br />
Lysozyme<br />
LYVE1 BROWN<br />
Mac 387 macrophage<br />
Mammaglobin<br />
MCPyV<br />
HHV8ip<br />
AHMB45<br />
AHPV<br />
AHSP70<br />
HSV1<br />
HSV2<br />
IDH1<br />
AIGA<br />
AIGD<br />
AIGG4<br />
AIGG4<br />
AIGM<br />
AINHIB<br />
AINSU<br />
ACD25<br />
AJ<br />
AKAPY<br />
AKAPPA<br />
Ki67<br />
Ki67<br />
APANKLAM<br />
LAMBDABM<br />
ALAMBDA<br />
ALAM<br />
ALAMY<br />
LANG<br />
LCAMH<br />
ACD15<br />
ALN3<br />
ALH<br />
ALYSO<br />
LYVE1 BROWN<br />
AMC387<br />
AMAMA<br />
MCPYV
Melan A/Melanoma<br />
MELANA<br />
Merosin Frozen<br />
MHC1<br />
AMHC1<br />
MITF DAB<br />
AMITFD<br />
Mitochondrial<br />
MITOC<br />
MLH1 Homolog<br />
MLH1<br />
MOC-31<br />
MOC31<br />
MSH2<br />
MSH<br />
MSH6<br />
AMSH6<br />
MUC-1<br />
AMUC1<br />
MUC-2<br />
AMUC2<br />
MUC-4<br />
AMUC4<br />
MUC-5AC<br />
AMUC5AC<br />
MUC-6<br />
AMUC6<br />
MUM-1<br />
AMUM1<br />
Myeloperoxidase<br />
AMPO<br />
Myogenin<br />
AMYOGN<br />
Myoglobin<br />
AMYO<br />
Myosin Heavy Chain<br />
ASMYOS<br />
N-Cadherin<br />
Napsin A<br />
ANAPA<br />
NEUN<br />
ANEUNUC<br />
Neur<strong>of</strong>ilament<br />
ANFIL<br />
Neuron Specific Enolase<br />
ANSE<br />
Neutrophil Elastase<br />
ANEUNUC<br />
NKIC3 melanoma<br />
ANKIC3<br />
OCT_2<br />
Oct_2<br />
OCT3/4<br />
OCT3/4<br />
OPD4 (CD45RO)<br />
AOPD4<br />
Osteocalcin<br />
AOC<br />
Osteonectin<br />
AON<br />
P16<br />
AP16<br />
P21, WAF1 AP21<br />
P27<br />
AP27<br />
P40<br />
AP40
P501S<br />
P504S<br />
P53 Tumor Suppressor Protein<br />
P63 Tumor Suppressor Protein<br />
P75 NGF<br />
P903<br />
Pan Cytokeratin<br />
Pancreatic Polypeptide<br />
PANK (for PANK/LAM DS)<br />
Parathyroid Hormone<br />
Parvalbumin<br />
Parvovirus<br />
Pax-5, B-cell Specific Activator Protein<br />
PAX-8<br />
PD1<br />
PGP 9.5 Neuroendocrine<br />
PIN4<br />
Plakoglobin<br />
PLAP<br />
PMAC P5B<br />
PMS2<br />
Pneumocystis carinii<br />
PPARgamma<br />
PREA ATTR<br />
Progesterone Receptor<br />
Prolactin<br />
Prostate Specific Acid Phosphatase<br />
Prostatic Specific Antigen<br />
Prostate Specific Membrane Antigen<br />
PSTAT3<br />
Renal Cell Carcinoma<br />
Respiratory Syncytial Virus<br />
S100<br />
Sarcomeric Actin<br />
Serotonin<br />
P501S<br />
AP504S<br />
AP53<br />
AP63<br />
P75<br />
AP903<br />
CKPAN<br />
APP<br />
APANKLAM<br />
APTH<br />
APARV1<br />
APARVO<br />
APAX5<br />
PAX8<br />
PD1<br />
APFP<br />
APIN4<br />
PLAP<br />
PMAC<br />
PMS2<br />
APCAR<br />
PPARg<br />
ATTR<br />
APR<br />
APRO<br />
APAP<br />
APSA<br />
PSMA<br />
APSTAT<br />
ARCC<br />
ARSV<br />
AS100<br />
ASACT<br />
ASERO
SMAD4<br />
Smoothelin<br />
Somatostatin<br />
SOX11<br />
Surfactant Protein A<br />
SV40 papova virus<br />
Synaptophysin<br />
TAG-72<br />
TAU<br />
Terminal Deoxynucleotide Transferase - TdT<br />
TDP43<br />
TFE3<br />
Thrombomodulin<br />
Thyroglobulin<br />
TIA1 granzyme<br />
Toxoplasma<br />
Treponema pallidum<br />
Trypsin<br />
TRYPTASE<br />
TSH<br />
TTF-1<br />
Tyrosinase<br />
Ubiquitin<br />
UCHL1 CD45RO<br />
Ulex Europaeous Lectin<br />
UP III<br />
Varicella zoster<br />
VEGF, Vascular Endothelial Growth Factor<br />
Vimentin<br />
WT-1<br />
SMAD4<br />
SMOOTHELIN<br />
ASOMAT<br />
SOX11<br />
ASPA<br />
ASV40<br />
ASYNP<br />
ATAG<br />
TAU<br />
TDT<br />
TDP-43<br />
TFE3<br />
ATHROMBO<br />
ATHYRO<br />
ATIA1<br />
ATOXO<br />
ATREP<br />
TRYP<br />
TRYPTASE<br />
ATSH<br />
TTF1<br />
ATYROS<br />
AUBQ<br />
AUCHL1<br />
AULEX<br />
AUPIII<br />
AVZV<br />
AVEGF<br />
AVIMEN<br />
AMESO
CODES FOR DOUBLE LABELING<br />
ICKDE<br />
AE1/AE3 and desmin<br />
ICKAC<br />
ICKMI<br />
AE1/AE3 and actin (HHF35)<br />
AE1/AE3 and MIB-1<br />
In all cases cytokeratin staining will be red (AEC) and the second antibody will be black (Nickel enhanced<br />
DAB).<br />
IKPLM<br />
Kappa (black) and Lambda (red)<br />
IBT<br />
T-cell (CD3-black) and B-cell (L26/CD20-red)<br />
IN-SITU HYBRIDIZATION PROBES AVAILABLE<br />
Insitu (Brightfield)<br />
iADV Adenovirus<br />
iBKV BKV insitu<br />
iCMV CMV insitu<br />
iEBER EBER probe for EBV mRNA (Ventana)<br />
iHPV HPV probe panel (manual method)<br />
i1618 HPV 16,18,31,33,35,39,45,51,52,56,58,66 subtype (high)(Ventana)<br />
i611<br />
HPV 6+11 subtype (low) (Ventana)<br />
iHSV HSV probe<br />
iJCV<br />
JC virus probe<br />
iRLAM mRNA for Lambda light chain restriction (Ventana)<br />
iRKAP mRNA for Kappa chain restriction (Ventana)<br />
IHC testing in ISH Laboratory<br />
aMIT Mitochondrial antibody<br />
aPALABU Kidney<br />
aBAPP Neuropathology
IHC/ISH Reporting<br />
Templates
PHS/B:______________________<br />
PARAFFIN SECTION IMMUNOHISTOCHEMISTRY/IN-SITU HYBRIDIZATION<br />
Name:______________________<br />
In order to characterize ___________________, paraffin section immunohistologic/in-situ hybridization studies were performed on<br />
___________________.<br />
The following results were found:<br />
Antigen/Antibody<br />
anti-kappa<br />
kappa mRNA-ISH<br />
anti-lambda<br />
lambda mRNA-ISH<br />
anti-IgG<br />
anti-IgG4<br />
anti-IgA<br />
anti-IgM<br />
anti-IgD<br />
CD20/L26<br />
CD22<br />
CD79a<br />
PAX 5<br />
CD21<br />
CD23<br />
CD138<br />
J chain<br />
BCL2<br />
BCL2 E17<br />
MYC<br />
BCL6<br />
CD10<br />
IRF4/MUM-1<br />
Cyclin D1<br />
SOX-11<br />
P27<br />
LEF-1/TCF-1<br />
Annexin A1<br />
TdT<br />
CD45/LCA<br />
CD15/Leu M1<br />
CD30/Ber H2<br />
EMA<br />
OCT-2<br />
Bob.1<br />
ALK-1<br />
Clusterin<br />
CD1a<br />
CD2<br />
CD3<br />
CD4<br />
B-cell subset<br />
B-cell subset<br />
B-cell subset<br />
B-cell subset<br />
B-cell subset<br />
B-cell subset<br />
B-cell subset<br />
B-cell subset<br />
B-cell subset<br />
B-cells<br />
B-cells<br />
B-cells<br />
B-cells<br />
Follicular dendritic cells<br />
B-cell subset, follicular dendritic cells<br />
Plasma cells, other<br />
Plasma cells, other<br />
Lymphocyte subset, other<br />
Lymphocyte subset, other<br />
MYC protein<br />
Follicular center cells, other<br />
B-cell subset<br />
B-cell subset<br />
Mantle cell lymphoma, other<br />
Mantle cell lymphoma, other<br />
Lymphoid subset<br />
CLL/SLL, T-cells, other<br />
Hairy cell leukemia, myeloid, T cell<br />
subset<br />
Lymphoblasts, some myeloblasts<br />
Leukocytes<br />
Reed-Sternberg cells, myeloid<br />
RS cells, activated lymphs<br />
Epithelium, lymphocyte subset<br />
B-cells, other<br />
B-cells, other<br />
Anaplastic large cell lymphoma kinase<br />
ALCL, other<br />
Thymocytes, Langerhans-type cells<br />
T and NK-cells<br />
T and NK-cells<br />
T-helper/inducer cells<br />
Result
CD5<br />
CD7<br />
CD8<br />
CD43/Leu 22<br />
CD279/PD-1<br />
CXCL13<br />
Beta-F1<br />
Gamma TCR<br />
CD56<br />
CD57/Leu 7<br />
TIA1<br />
Granzyme B<br />
CD25<br />
CD68/PGM-1<br />
CD123<br />
TCL-1<br />
Myeloperoxidase<br />
Lysozyme<br />
CD14<br />
CD163<br />
CD33<br />
Neutrophil elastase<br />
CD117<br />
Tryptase<br />
CD34<br />
Glycophorin<br />
E-cadherin<br />
T-cells, B-cell subset<br />
T and NK-cells<br />
T-cytotoxic/suppressor cells<br />
T-cells, some B-cells, myeloid<br />
T-follicular helper cells, other<br />
T-follicular helper cells, other<br />
TCR-beta chain<br />
TCR gamma<br />
T-cell subset and NK-cells<br />
T and NK cell subsets<br />
Cytotoxic cells<br />
Activated cytotoxic cells<br />
Activated lymph, other<br />
Myeloid, macrophages<br />
Plasmacytoid dendritic cells,<br />
Hairy cell leukemia, other<br />
Plasmacytoid dendritic cells, T-PLL,<br />
B-cell subset, other<br />
Myeloid<br />
Myeloid, macrophages<br />
Monocytic cells/macrophages<br />
Monocytic cells/macrophages<br />
Myeloid, macrophages, mast cells<br />
Myeloid<br />
Mast cells, myeloid precursors, other<br />
Mast cells<br />
Progenitor cells, endothelial cells, other<br />
Erythroid<br />
Immature erythroid, other<br />
Factor VIIIRA Megakaryocytes, endothelial cells<br />
CD61<br />
Megakaryocytes<br />
Ki-67/MIB-1 Proliferating cells<br />
P53<br />
Tumor suppressor gene<br />
AE1/AE3<br />
Cytokeratin<br />
Cam 5.2<br />
Cytokeratin<br />
Pankeratin<br />
Cytokeratin<br />
S100/S100a Neural, melanoma & other<br />
CD207/Langerin Langerhans cells<br />
EBV-ISH (EBER) Epstein-Barr virus<br />
EBV-LMP<br />
Epstein-Barr virus<br />
Parvovirus<br />
Parvovirus<br />
CMV<br />
Cytomegalovirus<br />
HHV8<br />
Human herpes virus-8<br />
H.Pylori<br />
H. Pylori<br />
B. henselae Bartonella henselae<br />
HSV 1/2 Human herpes viruses 1 & 2
Molecular Diagnostic and Cytogenetic Testing<br />
MOLECULAR DIAGNOSTIC TEST ORDERING:<br />
MOLECULAR DIAGNOSTICS WEBSITE:<br />
http://path.upmc.edu/divisions/mdx/diagnostics.html<br />
<br />
<br />
<br />
printable requisition form<br />
information on required sample types for each test<br />
frequently asked questions are answered<br />
Request on BM specimens should be given to BM technologists – message can be left on the Audix line (802-<br />
3273). On lymph node service, please fax requisition and save with fax “receipt” in notebook in room G323. It<br />
is also preferred that you call to inform them a fax is coming. Lymph node assistant or backup should help.<br />
The molecular oncology testing schedule (after DNA/RNA preparation) is as follows (updated 1/2012; subject<br />
to change per MDX policy)<br />
Monday/Thursday: TCR, IgH, JAK2, BCL-2 PCR testing is started and results out the next day<br />
Wednesday: Quantitative BCR-ABL and Quantitative PML testing is started and results out Thursday or Friday<br />
Wednesday: TCR, IgH, BCL-2 Southern blot testing is started and results out the following Tuesday<br />
Please see below for example <strong>of</strong> requisition form.<br />
Additional Testing Information: Specialty labs will do PCR for B. henselae from frozen specimens or<br />
paraffin sections (test is not validated for paraffin sections). Their number is 800-421-7110, Pacific<br />
Time, if you have specific questions. The samples should be sent through Molecular Diagnostics, as<br />
for TB. Testing for Whipple disease can be arranged through the molecular diagnostics laboratory<br />
also.<br />
CYTOGENETICS TEST ORDERING: Please be sure that the pathology requisition is attached to the<br />
cytogenetics requisition and that the cytogenetic requisitions have at least the following information:<br />
• Name <strong>of</strong> the patient and numerical identifier<br />
• Pathologist’s name<br />
• Clinician’s name (if clear on requisition not necessary to repeat)<br />
• Reason for sending specimen (“r/o lymphoma” should be fine for all <strong>of</strong> our specimens unless<br />
you have different or more specific information to provide)<br />
Please see below for example <strong>of</strong> cytogenetics requisition form as well as testing menu
PITTSBURGH CYTOGENETICS LABORATORY<br />
Oncology Cytogenetic Study Requisition form<br />
PATIENT INFORMATION (Please Print)<br />
REFERRING PHYSICIAN (Please Print)<br />
Last Name: First: M.I.: Name:<br />
Address:<br />
City, State, Zip:<br />
Birthdate: ___________________ Sex: ______ Male ______<br />
Female<br />
Social Security #: ___________________<br />
Address:<br />
City, State, Zip:<br />
Telephone:<br />
Medical Record #:<br />
Account#:<br />
Inpatient? _____ Location: _______________ Outpatient? _____<br />
Specimen information:<br />
Date/Time <strong>of</strong> Collection: _______________ Amount Drawn: ________<br />
Type <strong>of</strong> Specimen:<br />
_____ Bone Marrow<br />
_____ Peripheral Blood (unstimulated)*<br />
_____ Tumor type (location)__________________________<br />
_____ Lymph Node (location)_________________________<br />
* Peripheral blood may be submitted for unstimulated studies only if<br />
circulating blast count is above 5%.<br />
____Pre-Bone Marrow Transplant Sex <strong>of</strong> Donor: ____Male ____<br />
Female<br />
____ Post-Bone Marrow Transplant #days _____<br />
Fax:<br />
Additional Report To:<br />
Address:<br />
City, State, Zip:<br />
Phone:<br />
Fax:<br />
Clinical History/Pertinent Physical Findings (include<br />
chemotheraphy and radiation therapy dates/drugs):<br />
Signature <strong>of</strong> Requesting Physician (REQUIRED!):<br />
INDICATION FOR STUDY: (MUST BE COMPLETED!) * CIRCLE ALL THAT APPLY *<br />
Anemia Thrombocytopenia Leukocytosis Leukopenia Pancytopenia Lymphoma Other (Specify)<br />
Diagnosis: Tentative Confirmed New Diagnosis? Remission Sample? Relapse Sample?<br />
CML: Chronic Phase Blast Phase ALL: B-ALL T-ALL CLL MM MPD (Specify)<br />
MDS AML Other (Specify)<br />
TEST(S) REQUESTED: (MUST BE COMPLETED!) * CIRCLE ALL THAT APPLY *<br />
Chromosome Analysis (Karyotype) Yes No<br />
Fluorescence in-situ hybridization (FISH) panels requested: * CIRCLE ALL THAT APPLY *<br />
MDS Panel MPD Panel AML Panel CLL Panel MM Panel Children’s ALL<br />
Panel<br />
FLUORESCENCE IN SITU HYBRIDIZATION (FISH)<br />
B-Cell lymphoma<br />
Panel<br />
Monosomy/Trisomy Translocation/fusion Break-apart rearrangement Solid tumors<br />
___5q- ___BCR/ABL t(9;22) ___ALK (2p23) ___MLL (11q23) ___12CEP/12p<br />
___Monosomy 7/ 7q- ___BIRC3/MALT t(11;18) ___AML1 (21q22) ___MYC (8q24) ___1p36/19q<br />
___Trisomy 8 ___CBFβ/MYH1 inv(16) ___BCL2 (18q21) ___PAX5 (9p13) ___ALK (2p23)<br />
___Trisomy 9 ___ETV6/RUNX1 t(12;21) ___BCL3 (19q13)<br />
___Trisomy 12 ___IGH@/FGFR3 t(4;14) ___BCL6 (3q27))<br />
___PDGFRB (5q33)<br />
___RARA (17q21)<br />
___TCF3 (19p13)<br />
___TCL1 (14q32)<br />
___CHOP (12q13)<br />
___ERBB2/CEP17<br />
___13q- ___IGH@/BCL2 t(14;18) ___BCL10 (1p22) ___TCRAD (14q11) ___EWSR1 (22q12)<br />
___20q-<br />
___Hyperdiploidy 5, 7, 9<br />
___ATM (11q-)<br />
___CMYB (6q-)<br />
___P16(CDKN2A)<br />
(9p21)<br />
___IGH@/CCND1 t(11;14)<br />
___CCND1 (11q13)<br />
___EVI1 (MECOM) (3q26)<br />
___TCRB (7q34)<br />
___FKHR(FOXO1)<br />
(13q14)<br />
___IGH@/MAF t(14;16) ___FGFR1 (8p12) ___TCRG (7p14) ___MONOSOMY 3<br />
___IGH@/MALT1 t(14;18) ___IGH (14q32) ___TEL(ETV6) (12p13) ___N-MYC<br />
___IGH@/MYC t(8;14) ___IGK (2p11) ___TLX1 (10q24) ___SYT (18q11.2)<br />
___PML/RARA t(15;17) ___IGL (22q11) ___TLX3 (5q35) ___TLS(FUS) (16p11.2)
PITTSBURGH CYTOGENETICS LABORATORY<br />
Oncology Cytogenetic Study Requisition form<br />
___P53 (del 17p13.1)<br />
___RUNX1T1/RUNX1<br />
t(8;21)<br />
___MALT1 (18q21<br />
___PAX5 (del 9p13)<br />
___XX/XY<br />
Updated: 3/25/13 JH<br />
___CHIC2/FIP1L1-<br />
PDGFRA (4q12)<br />
Others (Specify):_______________________________________________________________________________<br />
Magee-Womens Hospital <strong>of</strong> UPMC<br />
300 Halket St., Rm. 1225<br />
Pittsburgh, PA 15213<br />
(412)641-5558 PHONE (412)641-2255 FAX
Cytogenetics Test Menu<br />
CYTOGENETICS Blank Slides Available Order Blanks - See Page One<br />
Break Apart Rearrangement Probes<br />
Other<br />
ALK (2p23) RARA (17q21) ASS deletion (9q34)<br />
AML1 (21q22) TCRB (7q34) ATM deletion (11q22.3)<br />
BCL2 (18q21) TCRG (7p14) CEP X/Y<br />
BCL3 (19q13) TCRAD (14q11) D7S486/CEP7 -7/7q31 I (7q)<br />
BCL6 (3q27) TCR3 (19p13) (D7S522/CEP7) - 7/7q31<br />
BCL10 (1p22) TCL1(14q32.1) Deletion 13q (13q14) D135319<br />
CBFB (16q22)<br />
Deletion 20q (20q12) D205108<br />
CCND1 (11q13) Translocation Probes EGR1 -5/5q-<br />
ETV6 (TEL) (12p13) RUNX1T1/RUNX1 (ETO/AML1) t(8;21) AML FIP1L1-CHIC2-PDGFRA(4q12) (CHIC2 deletion)<br />
EVI1 (MECOM) (3q26.2) BIRC3-MALT1 (API2-MALT1) t(11;18) CDKN2A (p16) (9p21 deletion)<br />
EWSR1 (22q11.2) BCR-ABL t(9;22) CML/ALL/AML TP53 deletion (17p13.1)<br />
FGFR1 (8p12) IGH - CCND1 (T11:14) Trisomy 5, 9, 15<br />
IGH (14q32) IGH-BCL2 t(14;18) Trisomy 8 D8Z2<br />
IGK (2p11) CBFB-MYH1 (16p13/16q22) t(16;16). inv(16) Trisomy 12 D1273<br />
IGL (22q11)<br />
IGH-FGFR3 t(4;14)<br />
MALT1 (18q21)<br />
IGH-MAF t(14;16)<br />
MLL (11q23)<br />
IGH MALT1 t(14;18)<br />
MYC (8q24)<br />
IGH-MYC t(8;14)<br />
MYCN (2P23-24) (for amp)<br />
PML-RARA t(15;17)<br />
PAX5 (9p13)<br />
TEL-AML1 (ETV6/RUNX1) t(12;21)<br />
PDGFRB (5q33)<br />
Panels<br />
B-Cell Lymphoma - BCL6 (3q27) / MYC (8q24) / IGH (14q32.3) / BCL2 (18q21)<br />
CLL - MYB (6q23) / ATM (11q22.3) / trisomy 12 / D13S319 (13q14.3) / IGH (14q32.3) / p53 (17p13.1)<br />
Multiple Myeloma MM - D13S319 (13q14.3) / ATM (11q22.3) / p53 (17p13.1) / IGH (14q32.3) / CCND1 (11q13)<br />
Childrens' ALL - CEP4/CEP10/CEP17 (trisomies 4, 10, 17) / BCR/ABL [t(9;22)] / MLL (11q23) / TEL-AML1 (ETV6/RUNX1) t((12;21)<br />
Childrens' AML - EGR1 (5q31) / monosomy 7 / ETO-AML1 (RUNX1T1/RUNX1) [t(8;21)] / MLL (11q23) / CBFB [inv(16)]<br />
MDS/AML - EGR1 (5q31) / D7S486 (7q31)-CEP7 / trisomy 8 / D2OS108 (20q12)<br />
AML Panel - EGR1 (5q31) / D7Z1 - D7S486 / RUNX1T1-RUNX1[t(8;21)] / CBFB [inv(16) or t(16;16)] / MLL [t(11;var)]<br />
[as needed (i.e. if not seen by classical analysis)]<br />
MPD panel - BCR-ABL1 / CEP8 / CEP9 / D2OS108 (20q12)<br />
Myeloid/lymphoid neoplasm with eosinophilia - FIP1L1-CHIC2-PDGFRA (4q12), PDGFRB (5q33), FGFR1 (8p13)<br />
Additional FISH Probes Request (please list) Requested Ordered Blanks Sent<br />
Comments
FISH panels for Hematologic Malignancies<br />
CLL Panel<br />
DNA Probe<br />
Chromosome Breakpoint<br />
1. D13S319/LAMP2 13q14.3<br />
2. CEP 12 12p11.1-q11.1<br />
3. ATM 11q22.3<br />
4. p53 17p13.1<br />
5. CMYB 6q23<br />
6. IGH 14q32.3*<br />
*If IGH is positive, we may recommend a few translocation probes involving 14q32<br />
a. IGH/BCL2 t(14;18)(q32;q21)<br />
b. IGH/CCND1 t(11;14)(q13;q32)<br />
Multiple Myeloma (MM) Panel<br />
DNA Probe<br />
Chromosome Breakpoint<br />
1. IGH 14q32.3<br />
2. IGH/CCND1 14q32/11q13<br />
3. TP53/CEP17 17p13.1<br />
4. D13S319/LAMP1 13q14.3<br />
5. D5S23/D5S721,9q34,CEP7 5p15.2, 9q34, 7p11.1-q11.1<br />
(hyperdiploidy)<br />
If IGH is + and IGH/CCND1 is -, then we will do FISH for:<br />
IGH/FGFR3 t(4;14)(p16;q32)<br />
IGH/MAF t(14;16)(q32;q23)<br />
If classical chromosome analysis is normal and the above MM FISH panel is negative, then the<br />
following FISH will be performed reflexively:<br />
ALL panel<br />
DNA Probe<br />
Chromosome Breakpoint<br />
1. BCR/ABL 9q34/22q11.2<br />
2. MLL 11q23<br />
3. ETV6/RUNX1 12p13/21q22<br />
(TEL/AML1)<br />
4. CEP4 4p11-q11<br />
5. CEP10 10p11.1-q11.1<br />
6. CEP17 17p11.1-q11.1
B-Cell Lymphoma Panel<br />
DNA Probe<br />
Chromosome Breakpoint<br />
1. BCL6 3q27<br />
2. MYC 8q24<br />
3. IGH 14q32<br />
4. BCL2 18q21<br />
MDS Panel<br />
DNA Probe<br />
Chromosome Breakpoint<br />
1. EGR1 5q31<br />
2. CEP 7 7p11.1-q11.1<br />
3. D7S486 7q31<br />
4. CEP 8 8p11.1-q11.1<br />
5. D20S108 20q12<br />
MPD Panel<br />
DNA Probe<br />
Chromosome Breakpoint<br />
1. BCR/ABL 9q34/22q11.2<br />
2. CEP 8 8p11.1-q11.1<br />
3. CEP 9 9p11-q11<br />
4. D20S108 20q12<br />
AML Panel<br />
DNA Probe<br />
Chromosome Breakpoint<br />
1. D7Z1/D7S486 7p11.1-q11.1/7q31<br />
2. EGR1 5q31/5p15.2<br />
3. RUNX1T1/RUNX1 8q22/21q22<br />
(ETO/AML1)<br />
4. CBFB 16q22 [inv(16)]<br />
5. MLL 11q23<br />
MALT Lymphoma Strategy<br />
DNA Probe Chromosome Breakpoint<br />
1. MALT1 18q21*<br />
*If MALT1 is positive, we will recommend a few translocation probes involving<br />
18q21<br />
a. IGH/MALT1 t(14;18)(q32;q21)<br />
b. API2/MALT1 t(11;18)(q21;q21)
CML Strategy<br />
ES probe<br />
1. BCR/ABL ES t(9;22)(q34;q11.2)*<br />
*If the extra signal is not observed using the BCR/ABL ES DNA probe and the cells are<br />
positive for the fusion signal in a considerable proportion <strong>of</strong> cells, perform FISH using the<br />
LSI ASS (9q34) DNA probe to detect a deletion <strong>of</strong> 9q.<br />
*If there is suspicion for the acute phase or blast phase <strong>of</strong> CML, we may suggest the<br />
following FISHes to detect trisomy 8 and/or i(17q) using the following DNA probes:<br />
a. CEP 8 8p11.1-q11.1<br />
b. RARA/CEP17 17q21
CoPath and Dictation<br />
Pointers
COPATH Related Instructions for Dictation <strong>of</strong> Final Reports<br />
1. At time <strong>of</strong> dictating any report, the patient name, the CoPath pathology report number,<br />
the medical record number should be stated in all instances except the occasional<br />
consult in which this information is not available. With the exception <strong>of</strong> the other cases,<br />
the number put in the dictaphone should be the MR number. At the end <strong>of</strong> the dictation,<br />
state that this is the end <strong>of</strong> the dictation on ____________ (given patient’s name).<br />
2. The trainee dictating at the end <strong>of</strong> the final diagnosis can state “do not mark this case<br />
complete.” In this instance, the transcriptionist will not finalize the case by marking it<br />
“complete” during the transcription process. If the report is dictated before noon, within<br />
two hours it will be available for correction by the trainee. If it is dictated afternoon, four<br />
hours later, the report will be available for corrections. In all instances, reports dictated<br />
before the four o’clock cut-<strong>of</strong>f would be transcribed on the same day. The trainee then<br />
can go into the report and edit the final diagnosis and microscopic (as well as the gross<br />
and clinical history they choose) and when finished they must mark the case as<br />
“complete” which will then sent to the physician’s electronic sign-out queue. During this<br />
process the trainee is to verify that the final diagnosis matches that written on the hard<br />
copy during sign-out. If a report is not ready, contact the secretarial supervisor. The<br />
trainee should then give the paperwork including a printed final copy to the staff<br />
pathologist.<br />
The trainee should ask the faculty person if the above is what they want. If not, be sure to<br />
given the faculty person all paperwork.<br />
3. All “UP” cases (white or yellow sheet consults) must have a billing code dictated. These<br />
consult cases have either a white or yellow sheet on the folder containing the consult<br />
case. Blue sheet consults (usually performed at request <strong>of</strong> clinician) do not need a<br />
billing code dictated.<br />
4. Cut <strong>of</strong>f times:<br />
Cases dictated by 5:00 PM Monday – Friday will be typed that day<br />
Cases dictated after 5:00 PM Monday – Friday will be typed by 10:00 AM the<br />
next day<br />
Saturday: cases dictated by 12 Noon will be typed that day<br />
BILLING CODES FOR “UP” CASES (“WHITE” OR “YELLOW” SHEET CONSULTS)<br />
BC#<br />
Consultation Type<br />
1 Level I Consult (very simple review, no extra stains)<br />
2 Level II Consult (greater than 4 H&E, or up to 3 IHC stains)<br />
3 Level III Consult (4-7 IHC stains)<br />
4 Level IIII Consult (complex with 8-11 IHC stains)<br />
5 Level V Consult (complex with ≥12 IHC stains)<br />
6 Lymph Nodes with Flow Cytometry and No Immunostains<br />
14 No Charge (including VA flows)<br />
15 Stains, only
FLOW/IHC cases -- coded comments for discussion<br />
Coded comments to add to end <strong>of</strong> microscopic description after immunostain table or if<br />
there is no immunostain table (because it will be in an addendum) under “Ancillary<br />
Studies.”<br />
FLOW/IHC1: Medical necessity justification for the immunohistochemical stains that were<br />
needed in addition to the flow cytometric immunophenotypic studies for the best diagnosis<br />
possible is as follows: The flow cytometric studies did not define the precise nature <strong>of</strong> all the<br />
cellular elements <strong>of</strong> concern in this specimen.<br />
[to be used for example if a cyclin D1 stain with other markers is needed or if flow didn't<br />
evaluate a plasma cell or possible RS population]<br />
FLOW/IHC2: Medical necessity justification for the immunohistochemical stains that were<br />
needed in addition to the flow cytometric immunophenotypic studies for the best diagnosis<br />
possible is as follows: The flow cytometric studies are not clearly representative <strong>of</strong> all the<br />
features requiring evaluation in this specimen.<br />
[To be used for example if you have lymphoid aggregates in marrow that might not be in the<br />
flow suspension, suspension was dilute]<br />
Coded comment to add to end <strong>of</strong> flow report when it will precede the complete report<br />
with the above coded comments:<br />
FLOW1: Because these flow cytometric studies do not fully clarify the diagnosis in this case,<br />
immunohistochemical stains will be performed on this specimen and a final report will follow.
Division <strong>of</strong> <strong>Hematopathology</strong><br />
Dictating & Pro<strong>of</strong>ing Tissue Reports*<br />
(Suggestions for trainees)<br />
Patient history:<br />
Look at prior cases in CoPath worksheet, outside reports, requisitions, MARS (if patient<br />
is or has been at UPMC), letter (if consult) for historical information and be sure to<br />
include in report since, in part, some or all <strong>of</strong> this information may be very hard to find at<br />
a later date. This is OUR responsibility. For bone marrow cases, the technologists <strong>of</strong>ten<br />
enter a clinical history, but it is up to us to verify the accuracy and completeness.<br />
<br />
Be sure pre-op, post-op diagnoses and procedure have been entered.<br />
Diagnosis:<br />
Be sure to use standard format<br />
o Tissue type, tissue site, procedure (and if consult, Outside slide number “OSS….,<br />
institution”)- diagnosis using terminology <strong>of</strong> WHO (if malignant)<br />
NEVER use “consistent with” in a diagnostic line. This is departmental policy.<br />
If more than one specimen on a consult, the different parts should be in chronological<br />
order.<br />
Abbreviations should be avoided; if used in a report, the full term should be provided the<br />
first time it is used.<br />
Comment:<br />
Be sure that the comment includes the methods used to arrive at the diagnosis.<br />
Phenotypic aberrancies that might be useful to follow up on in any subsequent<br />
specimens are useful to include here. Ask the attending pathologist if there is any<br />
question about what should be included in the comment.<br />
The comment should stress the major diagnosis first and must be consistent with the<br />
diagnoses listed above (i.e.: it should not “back-track” on a definitive diagnosis).<br />
The comment does not necessarily have to follow the same order as the specimens<br />
listed in the diagnosis (i.e., a definitive diagnosis might be dealt with first).<br />
Be sure at least the comment, if not the diagnosis, clearly indicates any studies still<br />
pending at the time <strong>of</strong> signout. These should not come as a surprise to the physician<br />
receiving the report.<br />
Microscopic description<br />
Microscopic descriptions should “conjure up” and clearly convey an image that is<br />
consistent with the diagnosis. “Buzz words” can be used to help achieve brevity and,<br />
while it is best to avoid using descriptions to make conclusions, there is no need to<br />
describe all the features <strong>of</strong> obviously reactive follicles or T-zone nodules.<br />
Microscopic descriptions in general should start with a statement concerning the tissue<br />
type, the low magnification architectural features and then the relevant cytologic<br />
features. Special stains should then follow and then the IHC/ISH template (if any such<br />
stains have been performed).<br />
IHC tables should follow the part that has been described in multipart specimens –<br />
easiest way so it’s clear what they refer to.<br />
Cases with flow cytometric studies and immunostains MUST HAVE a FLOW/IHC1 or 2<br />
comment at the end <strong>of</strong> this section.
On consult cases with prior flow cytometric studies, the outside flow reports should at<br />
least be summarized including a statement as to which laboratory did the studies. Place<br />
after (or before) the IHC/ISH results.<br />
On “UP” consult cases, be sure to dictate a billing code at the end. Cases with flow plus<br />
just H&E sections get BC6. Our other UP cases with 4-7 special stains will be BC3,<br />
more complex cases will be BC4 (8-11 special stains), with the most complex cases with<br />
greater than 12 stains getting BC5.<br />
Synoptic reports<br />
A synoptic should be added to all bone marrow and solid tissue reports when a<br />
hematopathologic/lymphoid neoplasm is being diagnosed. Currently on the bone<br />
marrows, these are being added on first-time diagnoses only, although they do not need<br />
to be limited to those cases.<br />
There are currently four (4) synoptics used in <strong>Hematopathology</strong>.<br />
o Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders Synoptic<br />
o Gastrointestinal Lymphoma Resection Synoptic<br />
o Hodgkin Lymphoma Biopsy/Staging Synoptic<br />
o Non-Hodgkin’s Lymphoma Biopsy/Resection Synoptic<br />
Synoptic reports may be dictated using the templates included this manual. A<br />
hard copy is available in rooms G315 and G323. Alternatively, they can be manually<br />
entered in CoPath after case dictation.<br />
Instructions for the use <strong>of</strong> synoptics are available online. The CoPath user guide is<br />
posted on the CoPathPlus website http://copath.upmc.com under the link for CoPathPlus<br />
Training Resources. The Synoptic Data Entry and Reporting Chapter is Chapter 24.<br />
Pro<strong>of</strong>reading<br />
Pro<strong>of</strong>read reports carefully and MAKE SURE that what is written not only is what was<br />
intended at sign out but that it makes sense. If pro<strong>of</strong>ing prior to giving to a faculty<br />
member and something doesn’t make sense, at least communicate that to them.<br />
Be sure to pro<strong>of</strong> numbers that have been included (including in the flow reports)<br />
Be sure that immunostain designations are what you intended (i.e., sometimes<br />
CD45RO/UCHL1 gets entered instead <strong>of</strong> CD45/LCA).<br />
Be sure singular/plural forms <strong>of</strong> words are correct, reports say “and” where you meant<br />
“and” and not “in”, “intra-“and “inter-“are used appropriately.<br />
Be sure the templated tables don’t have capital letters in the middle <strong>of</strong> a sentence.<br />
Unless directed otherwise, give the faculty member a printed out final version <strong>of</strong> your<br />
final report – not something with handwriting or a draft.<br />
Be sure to make an arrangement with the faculty member to see what changes were<br />
made in what you considered to be a final report.<br />
[Revised 10/2013]
Wording for Provisional Reports on Lymph Node Biopsies<br />
Provisional reports may be when there is a delay in issuing a complete final report. These are<br />
now a type <strong>of</strong> “special procedure”. These are to be used only on rare occasions.<br />
When dictating the case, the transcriptionist must be instructed to type a provisional report.<br />
Be sure the comment includes the following: This represents a provisional report. It will be<br />
replaced by a final diagnostic report once…<br />
<strong>Hematopathology</strong> Case Worksheet in CoPath<br />
1. Log into CoPath.<br />
2. From the top menu bar, choose FILE.<br />
3. Choose BROWSE ITEMS.<br />
4. Look in the Browse Items menu list. Scroll down until you get to HEME WORKSHEET.<br />
5. Click and drag to your menu on the left so that this report will be available to you the next<br />
time you log in.<br />
6. Double click on HEME WORKSHEET. The report will take 1 minute to load.<br />
7. Type the specimen number in the box under "Select a Specimen."<br />
8. Click on ADD.<br />
9. Run the report by clicking on OK.<br />
10. Click on PRINT.<br />
<strong>Resident</strong>/fellows can now review and print out the results <strong>of</strong> the special procedures that<br />
have been signed out by following the directions given below. The print out will also<br />
include the original diagnosis.<br />
Special Procedure Review by <strong>Resident</strong>/<strong>Fellow</strong> in CoPath<br />
1. Log into CoPath.<br />
2. From the top menu bar, choose FILE.<br />
3. Choose BROWSE ITEMS.<br />
4. Look in the Browse Items menu list. Scroll down until you get to PROCEDURE REVIEW BY<br />
RESIDENT/FELLOW.<br />
5. Click and drag to your menu on the left so that this report will be available to you the next<br />
time you log in.<br />
6. Double click on PROCEDURE BY RESIDENT/FELLOW.<br />
7. Choose the data field criteria desired to run the report:<br />
Typically for the data field:<br />
Choose the time range or date for Sign Out Date.<br />
For Specimen Class choose ALL VALUES<br />
<br />
<br />
For Procedure choose ALL VALUES<br />
For Person, click in the circle next to Individual Items, highlight the name <strong>of</strong> the<br />
resident/fellow, then click on ADD. (You can add multiple names.)<br />
8. Run the report by clicking OK.<br />
9. The next time you log in to CoPath, you can just choose the PROCEDURE BY<br />
RESIDENT/FELLOW report from your menu on the left and eliminate steps 2 to 5 above.
SYNOPTICS
Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
A1<br />
A2<br />
A3<br />
A4<br />
A5<br />
A6<br />
A7<br />
B1<br />
B2<br />
B3<br />
B4<br />
B5<br />
C1<br />
C2<br />
C3<br />
C4<br />
- - - - - - - - - - - - - - - - MACROSCOPIC - - - - - - - - - - - - - -<br />
SPECIMEN TYPE/PROCEDURE**<br />
(check all that apply)<br />
Aspirate<br />
Biopsy<br />
Particle Preparation<br />
Blood film<br />
Cell block (Clot section)<br />
Touch imprint<br />
Not specified<br />
BIOPSY/ASPIRATE SITE**<br />
Not applicable<br />
Right posterior iliac crest<br />
Left posterior iliac crest<br />
Other (specify):<br />
Not specified<br />
ADEQUACY OF SPECIMEN**<br />
Satisfactory<br />
Limited<br />
Unsatisfactory<br />
See report<br />
D1<br />
D2<br />
D3<br />
D4<br />
D5<br />
D6<br />
D7<br />
D8<br />
D9<br />
D10<br />
D11<br />
D12<br />
D13<br />
D14<br />
- - - - - - - - - - - - - - - - MICROSCOPIC - - - - - - - - - - - - - - -<br />
W HO CLASSIFICATION** (check all that apply)<br />
Note: This is NOT the final diagnosis. Use final diagno<br />
/comment for therapeutic decisions.<br />
Myeloproliferative Neoplasms<br />
Chronic myelogenous leukemia, BCR-ABL+<br />
Chronic myelogenous leukemia, accelerated phase<br />
Chronic myelogenous leukemia, myeloid blast crisis<br />
Chronic myelogenous leukemia, lymphoid blast crisis<br />
Chronic myelogenous leukemia, blast crisis, NOS<br />
Chronic neutrophilic leukemia<br />
Chronic eosinophilic leukemia, NOS<br />
Mastocytosis<br />
Cutaneous mastocytosis<br />
Systemic mastocytosis<br />
Systemic mastocytosis with associated clonal,<br />
hematologic non-mast-cell lineage disease<br />
Aggressive systemic mastocytosis<br />
Mast cell leukemia<br />
Mast cell sarcoma<br />
Extracutaneous mastocytoma<br />
---W HO Classifications Continued on Next Page----<br />
This is a worksheet. It is NOT the final diagnosis.<br />
6.5
Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
W HO CLASSIFICATION (check all that apply) CONT'<br />
D15<br />
Polycythemia vera<br />
Myelodysplastic Syndromes<br />
D16<br />
Primary myel<strong>of</strong>ibrosis<br />
F1<br />
Refractory anemia<br />
D17<br />
Essential thrombocythemia<br />
F2<br />
Refractory neutropenia<br />
D18<br />
Myeloproliferative neoplasm, unclassifiable<br />
F3<br />
Refractory thrombocytopenia<br />
D19<br />
Possible myeloproliferative neoplasm, see report<br />
F4<br />
Refractory anemia with ringed sideroblasts<br />
D20<br />
Favor myeloproliferative neoplasm but must rule out<br />
F5<br />
Refractory cytopenia with multilineage dysplasia<br />
other possibilities, see report<br />
F6<br />
Refractory cytopenia with multilineage dysplasia and<br />
ringed sideroblasts<br />
Myeloid and Lymphoid Neoplasms with Eosinophilia and<br />
Abnormalities <strong>of</strong> PDGFRA, PDGFRB OR FGFR1<br />
Refractory anemia with excess blasts (RAEB)<br />
D21<br />
Myeloid and lymphoid neoplasms with PDGFRA<br />
F7<br />
RAEB-1<br />
rearrangement<br />
F8<br />
RAEB-2<br />
D22<br />
Myeloid neoplasms with PDGFRB rearrangement<br />
D23<br />
Myeloid and lymphoid neoplasms with FGFR1<br />
F9<br />
Myelodysplastic syndrome, unclassifiable<br />
rearrangement<br />
F10<br />
Myelodysplastic syndrome associated with isolated<br />
del(5q)<br />
Myelodysplastic/ Myeloproliferative Neoplasms<br />
F11<br />
Childhood myelodysplastic syndrome<br />
E1<br />
Chronic myelomonocytic leukemia<br />
F12<br />
Refractory cytopenia <strong>of</strong> childhood<br />
E2<br />
Atypical chronic myeloid leukemia, BCR-ABL-<br />
F13<br />
Possible myelodysplastic syndrome, see report<br />
E3<br />
Juvenile myelomonocytic leukemia<br />
F14<br />
Favor myelodysplastic syndrome but must rule out<br />
E4<br />
Myelodysplastic/myeloproliferative neoplasm,<br />
other possibilities, see report<br />
unclassifiable<br />
F15<br />
Myelodysplastic syndrome, classification pends<br />
E5<br />
Refractory anemia with ring sideroblasts associated<br />
cytogenetics<br />
with marked thrombocytosis<br />
---W HO Classifications Continued on Next Page----<br />
6.5<br />
This is a worksheet. It is NOT the final diagnosis.
Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
G1<br />
W HO CLASSIFICATION (check all that apply) CONT'<br />
Acute Myeloid Leukemias (AML)<br />
Acute myeloid leukemia, not otherwise classified<br />
G12<br />
G13<br />
AML with myelodysplasia - Without antecedent<br />
myelodysplastic syndrome<br />
AML with myelodysplasia - With unknown prior<br />
history<br />
G2<br />
G3<br />
G4<br />
G5<br />
G6<br />
G7<br />
G8<br />
G9<br />
G10<br />
G11<br />
Acute myeloid leukemia with recurrent genetic<br />
abnormalities<br />
AML witH t(8;21)(q22;q22); RUNX1-RUNX1T1<br />
AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22);<br />
CBFB-MYH11<br />
Acute promyelocytic leukemia with t(15;17)(q22;q12);<br />
PML-RARA<br />
AML with t(9;11)(p22;q23); MLLT3-MLL<br />
AML with t(6:9)(p23;q34); DEK-NUP214<br />
AML with inv(3)(q21q26.2) or t(3;3)(q21;q26.2);<br />
RPN1-EVI1<br />
AML (megakaryoblastic) with t(1:22)(p13;q13);<br />
RBM15-MKL1<br />
AML with mutated NPM1<br />
AML with mutated CEBPA<br />
Acute myeloid leukemia with myelodysplasia-related<br />
changes<br />
AML with myelodysplasia - Following a<br />
myelodysplastic syndrome or myelodysplastic<br />
syndrome/myeloproliferative disorder<br />
Therapy-related myeloid neoplasms<br />
G14 Therapy-related myeloid neoplasms c/w AML<br />
G15 Therapy-related myeloid neoplasms c/w MDS<br />
G16 Therapy-related myeloid neoplasms c/w MDS/MPN<br />
G17 Therapy-related myeloid neoplasm, NOS<br />
G18<br />
G19<br />
G20<br />
G21<br />
G22<br />
G23<br />
G24<br />
G25<br />
G26<br />
G27<br />
G28<br />
Acute myeloid leukemia not otherwise categorized<br />
AML with minimal differentiation<br />
AML without maturation<br />
AML with maturation<br />
Acute myelomonocytic leukemia<br />
Acute monoblastic and monocytic leukemia<br />
Acute erythroid leukemia<br />
Acute megakaryoblastic leukemia<br />
Acute basophilic leukemia<br />
Acute panmyelosis with myel<strong>of</strong>ibrosis<br />
Acute myeloid leukemia, final classification pends<br />
cytogenetic studies<br />
Myeloid sarcoma<br />
---W HO Classifications Continued on Next Page----<br />
6.5<br />
This is a worksheet. It is NOT the final diagnosis.
Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
W HO CLASSIFICATION (check all that apply) CONT'<br />
Myeloid proliferations related to Down syndrome<br />
H2<br />
B lymphoblastic leukemia/lymphoma with recurrent<br />
genetic abnormalities<br />
G29<br />
Transient abnormal myelopoiesis<br />
H3<br />
B lymphoblastic leukemia/lymphoma with<br />
G30<br />
Myeloid leukemia associated with Down syndrome<br />
t(9;22)(q34;q11.2); BCR-ABL1<br />
H4<br />
B lymphoblastic leukemia/lymphoma with t(v;11q23);<br />
G31<br />
Blastic plasmacytoid dendritic cell neoplasm<br />
MLL rearranged<br />
H5<br />
B lymphoblastic leukemia/lymphoma with<br />
Acute leukemias <strong>of</strong> ambiguous lineage<br />
t(12;21)(p13;q22) TEL-AML 1 (ETV6-RUNX1)<br />
G32<br />
Undifferentiated acute leukemia<br />
H6<br />
B lymphoblastic leukemia/lymphoblastic lymphoma<br />
G33<br />
Mixed phenotype acute leukemia with<br />
with hyperdiploidy<br />
t(9;22)(q34;q11.2); BCR-ABL1<br />
H7<br />
B lymphoblastic leukemia/lymphoma with hypodiploidy<br />
G34<br />
Mixed phenotype acute leukemia with t(v;11q23);<br />
(hypodiploid ALL)<br />
MLL rearranged<br />
H8<br />
B lymphoblastic leukemia/lymphoma with<br />
G35<br />
Mixed phenotype acute leukemia, B/myeloid, NOS<br />
t(5;14)(q31;q32) IL 3-IGH<br />
G36<br />
Mixed phenotype acute leukemia, T/myeloid, NOS<br />
H9<br />
B lymphoblastic leukemia/lymphoma with<br />
G37<br />
Natural killer (NK) cell lymphoblastic<br />
t(1;19)(q23;p13.3); E2A-PBX1 (TCF3-PBX1)<br />
leukemia/lymphoma<br />
H10<br />
B lymphoblastic leukemia/lymphoma, final<br />
classification pends cytogenetic studies<br />
Mixed phenotype acute leukemia, NOS<br />
H11<br />
T lymphoblastic leukemia/lymphoma<br />
G38<br />
Bilineal acute leukemia<br />
G39<br />
Biphenotypic acute leukemia<br />
Mature B-Cell Neoplasms<br />
G40<br />
Acute leukemia, uncertain lineage<br />
J1<br />
B-cell lymphoid neoplasm, not further classified<br />
J2<br />
Small B-cell lymphoid neoplasm, not further classified<br />
Precursor lymphoid neoplasms<br />
J3<br />
Chronic lymphocytic leukemia/small lymphocytic<br />
H1<br />
B lymphoblastic leukemia/lymphoma<br />
B lymphoblastic leukemia/lymphoma, NOS<br />
lymphoma<br />
---W HO Classifications Continued on Next Page----<br />
6.5<br />
This is a worksheet. It is NOT the final diagnosis.
Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
J4<br />
J5<br />
J6<br />
J7<br />
J8<br />
J9<br />
J10<br />
J11<br />
J12<br />
J13<br />
J14<br />
J15<br />
J16<br />
J17<br />
J18<br />
J19<br />
J20<br />
J21<br />
J22<br />
J23<br />
W HO CLASSIFICATION (check all that apply) CONT'<br />
B-cell prolymphocytic leukemia<br />
Waldenstrom macroglogulinemia<br />
Heavy chain diseases<br />
Alpha heavy chain disease<br />
Gamma heavy chain disease<br />
Mu heavy chain disease<br />
Splenic marginal zone lymphoma<br />
Hairy cell leukemia<br />
Splenic B-cell lymphoma/leukemia, unclassifiable<br />
Splenic diffuse red pulp small B-cell lymphoma<br />
Hairy cell leukemia-Variant<br />
Lymphoplasmacytic lymphoma<br />
Plasma cell myeloma<br />
Monoclonal gammopathy <strong>of</strong> undetermined significance<br />
(MGUS)<br />
Solitary plasmacytoma <strong>of</strong> bone<br />
Extraosseus plasmacytoma<br />
Plasma cell neoplasm, not further categorized<br />
Primary amyloidosis<br />
Extranodal marginal zone lymphoma <strong>of</strong><br />
mucosa-associated lymphoid tissue (MALT-lymphoma)<br />
Nodal marginal zone lymphoma<br />
Pediatric nodal marginal zone lymphoma<br />
Follicular lymphoma<br />
J24 Follicular lymphoma, Grade not defined<br />
J25 Follicular lymphoma - Grade 1-2<br />
J26 Follicular lymphoma - Grade 3<br />
J27 Follicular lymphoma - Grade 3A<br />
J28 Follicular lymphoma - Grade 3B<br />
J29 Pediatric follicular lymphoma<br />
J30 Mantle cell lymphoma<br />
J31 Diffuse large B-cell lymphoma (DLBCL), NOS<br />
J32 T-cell/histiocyte rich large B-cell lymphoma<br />
J33 Primary DLBCL <strong>of</strong> the CNS<br />
J34 Primary Cutaneous DLBCL, leg type<br />
J35 EBV positive DLBCL <strong>of</strong> the elderly<br />
J36 DLBCL associated with chronic inflammation<br />
J37 Lymphomatoid granulomatosis<br />
J38 Primary mediastinal (thymic) large B-cell lymphoma<br />
J39 Intravascular large B-cell lymphoma<br />
J40 ALK positive large B-cell lymphoma<br />
J41 Plasmablastic lymphoma<br />
J42 Large B-cell lymphoma arising in HHV8-associated<br />
multicentric Castleman disease<br />
J43 Primary effusion lymphoma<br />
J44 Burkitt lymphoma<br />
---W HO Classifications Continued on Next Page----<br />
6.5<br />
This is a worksheet. It is NOT the final diagnosis.
Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
W HO CLASSIFICATION (check all that apply) CONT'<br />
K16<br />
Lymphomatoid papulosis<br />
J45<br />
B-cell lymphoma, unclassifiable, with features<br />
K17<br />
Primary Cutaneous gamma-delta T-cell lymphoma<br />
intermediate between diffuse large B-cell lymphoma<br />
K18<br />
Primary cutaneous CD8 positive aggressive<br />
and Burkitt lymphoma<br />
epidermotropic cytotoxic T-cell lymphoma<br />
J46<br />
B-cell lymphoma, unclassifiable, with features<br />
K19<br />
Primary cutaneous CD4 positive small/medium T-cell<br />
intermediate between diffuse large B-cell lymphoma<br />
lymphoma<br />
and classical Hodgkin lymphoma<br />
K20<br />
Peripheral T-cell lymphoma, NOS<br />
K21<br />
Angioimmunoblastic T-cell lymphoma<br />
Mature T-Cell and NK-Cell Neoplasms<br />
K22<br />
Anaplastic large cell lymphoma, ALK positive<br />
K1<br />
T-cell prolymphocytic leukemia<br />
K23<br />
Anaplastic large cell lymphoma, ALK negative<br />
K2<br />
T-cell large granular lymphocytic leukemia<br />
K3<br />
Chronic lymphoproliferative disorder <strong>of</strong> NK-cells<br />
Hodgkin Lymphoma<br />
K4<br />
Aggressive NK-cell leukemia<br />
L1<br />
Nodular lymphocyte predominant Hodgkin lymphoma<br />
K5<br />
Systemic EBV positive T-cell lymphoproliferative<br />
Classical Hodgkin lymphoma<br />
disease <strong>of</strong> childhood<br />
L2<br />
Nodular sclerosis classical Hodgkin lymphoma<br />
K6<br />
Hydroa vacciniforme-like lymphoma<br />
L3<br />
Lymphocyte-rich classical Hodgkin lymphoma<br />
K7<br />
Adult T-cell leukemia/lymphoma<br />
L4<br />
Mixed cellularity classical Hodgkin lymphoma<br />
K8<br />
Extranodal NK/T-cell lymphoma, nasal-type<br />
L5<br />
Lymphocyte-depleted classical Hodgkin lymphoma<br />
K9<br />
Enteropathy associated T-cell lymphoma<br />
L6<br />
Classical Hodgkin lymphoma, not further categorized<br />
K10<br />
Hepatosplenic T-cell lymphoma<br />
L7<br />
Hodgkin vs non-Hodgkin lymphoma<br />
K11<br />
Subcutaneous panniculitis-like T-cell lymphoma<br />
K12<br />
Mycosis fungoides<br />
Histiocytic & Dendritic-Cell Neoplasms<br />
K13<br />
Sézary syndrome<br />
M1<br />
Histiocytic sarcoma<br />
K14<br />
Primary cutaneous CD30 positive T-cell<br />
M2<br />
Langerhans cell histiocytosis<br />
K15<br />
lymphoproliferative disorders<br />
Primary cutaneous anaplastic large cell lymphoma<br />
M3<br />
Langerhans cell sarcoma<br />
---W HO Classifications Continued on Next Page----<br />
6.5<br />
This is a worksheet. It is NOT the final diagnosis.
Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
M4<br />
M5<br />
M6<br />
M7<br />
M8<br />
M9<br />
N1<br />
P1<br />
P2<br />
P3<br />
P4<br />
P5<br />
Q1<br />
Q2<br />
Q3<br />
W HO CLASSIFICATION (check all that apply) CONT'<br />
Interdigitating dendritic cell sarcoma<br />
Follicular dendritic cell sarcoma<br />
Fibroblastic reticular cell tumor<br />
Indeterminate dendritic cell tumor<br />
Disseminated juvenile xanthogranuloma<br />
Dendritic cell sarcoma, not otherwise specified<br />
Other<br />
Malignant neoplasm, type cannot be determined<br />
Post-transplant lymphoproliferative disorders<br />
Plasmacytic hyperplasia<br />
Infectious mononucleosis-like PTLD<br />
Polymorphic PTLD<br />
Monomorphic PTLD (specify type):<br />
Classical Hodgkin-lymphoma type PTLD<br />
ANCILLARY STUDIES**<br />
Flow Cytometry-Phenotyping**<br />
Flow cytometry immunophenotype performed, see<br />
separate report<br />
Flow cytometry immunophenotype pending, report to<br />
follow<br />
Flow cytometry immunophenotype not performed<br />
R1<br />
R2<br />
R3<br />
S1<br />
S2<br />
S3<br />
T1<br />
T2<br />
T3<br />
U1<br />
U2<br />
U3<br />
U4<br />
U5<br />
Immunohistochemistry/In Situ Hybridization**<br />
Immunohistochemistry/ in situ hybridization performed,<br />
see microscopic description<br />
Immunohistochemistry/ in situ hybridization pending,<br />
see addendum<br />
Immunohistochemistry/ in situ hybridization not<br />
performed<br />
Classical Cytogenetic Studies**<br />
Classical cytogenetic studies performed, see separate<br />
report<br />
Classical cytogenetic studies pending, report to follow<br />
Classical cytogenetic studies not performed<br />
Cytogenetic FISH Studies**<br />
Cytogenetic FISH studies performed, see separate<br />
report<br />
Cytogenetic FISH studies pending, report to follow<br />
Cytogenetic FISH studies not performed<br />
Genotypic (Molecular) Studies**<br />
Genotypic (Molecular) studies performed, see separate<br />
report<br />
Genotypic (Molecular) studies pending, report to follow<br />
Genotypic (Molecular) studies not performed<br />
DNA stored,Genotypic (Molecular) studies not<br />
performed<br />
RNA stored,Genotypic (Molecular) studies not<br />
performed<br />
This is a worksheet. It is NOT the final diagnosis.<br />
6.5
Bone Marrow/Peripheral Blood Hematopoietic/Lymphoid Disorders<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
V1<br />
V2<br />
V3<br />
Cytochemical Stains**<br />
Cytochemical stains performed, see microscopic<br />
description<br />
Cytochemical stains pending, see addendum<br />
Cytochemical stains not performed<br />
Y1<br />
ADDITIONAL PATHOLOGIC FINDINGS<br />
Specify:<br />
EXTENT OF BONE MARROW /PERIPHERAL BLOO<br />
INVOLVEMENT<br />
W1 Acute leukemia/MDS (bone marrow) - % blasts W2<br />
Acute leukemia/MDS (peripheral blood) - % blasts W3<br />
Malignant lymphoma - approximately<br />
involved<br />
Multiple myeloma<br />
W4 Plasma cells 30%<br />
% <strong>of</strong> area<br />
OVERALL MARROW CELLULARITY**<br />
X1
Non-Hodgkins Lymphoma Biopsy/Resection Synoptic Template<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
A1<br />
A2<br />
A3<br />
A4<br />
A5<br />
- - - - - - - - - - - - - - - - MACROSCOPIC - - - - - - - - - - - - - -<br />
SPECIMEN TYPE**<br />
Lymphadenectomy (specify sites):<br />
Other (specify):<br />
Not specified<br />
Splenectomy<br />
Other extranodal (specify):<br />
D1<br />
D2<br />
- - - - - - - - - - - - - - - - MICROSCOPIC - - - - - - - - - - - - - - -<br />
HISTOLOGIC TYPE (W HO CLASSIFICATION)**<br />
Note: This is NOT the final diagnosis. Use final diagno<br />
/comment for therapeutic decisions.<br />
Histologic type cannot be assessed<br />
Non-Hodgkin lymphoma vs Hodgkin lymphoma<br />
B1<br />
B2<br />
B3<br />
B4<br />
B5<br />
B6<br />
C1<br />
C2<br />
C3<br />
C4<br />
C5<br />
PROCEDURE (select all that apply)**<br />
Fine needle aspiration<br />
Needle core biopsy<br />
Biopsy, other<br />
Resection<br />
Other (specify):<br />
Unknown<br />
TUMOR SITE (check all that apply)**<br />
Lymph node(s), site unknown<br />
Lymph node(s) - Specify site(s),<br />
Other tissue(s) - Specify site(s):<br />
Not specified<br />
Only one site biopsied, see above<br />
D3<br />
D4<br />
D5<br />
D6<br />
D7<br />
D8<br />
D9<br />
D10<br />
D11<br />
D12<br />
B-cell Lymphoma<br />
B-cell lymphoma, subtype cannot be determined<br />
B-cell lymphoma with high grade features<br />
B-lymphoblastic leukemia/lymphoma, NOS<br />
B lymphoblastic leukemia/lymphoma with recurrent<br />
genetic abnormalities<br />
B lymphoblastic leukemia/lymphoma with<br />
t(9:22)(q34;q11.2); BCR-ABL1<br />
B lymphoblastic leukemia/lymphoma with t(v;11q23);<br />
MLL rearranged<br />
B lymphoblastic leukemia/lymphoma with<br />
t(12;21)(p13;q22) TEL-AML 1 (ETV6-RUNX1)<br />
B lymphoblastic leukemia/lymphoma with hyperdiploidy<br />
B lymphoblastic leukemia/lymphoma with hypodiploidy<br />
(hypodiploid ALL)<br />
B lymphoblastic leukemia/lymphoma with<br />
t(5;14)(q31;q32) IL 3-IGH<br />
---Histologic Types Continued on Next Page----<br />
This is a worksheet. It is NOT the final diagnosis.<br />
6.2
Non-Hodgkins Lymphoma Biopsy/Resection Synoptic Template<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
D13<br />
D14<br />
D15<br />
D16<br />
D17<br />
D18<br />
D19<br />
D20<br />
D21<br />
D22<br />
D23<br />
D24<br />
D25<br />
D26<br />
D27<br />
D28<br />
D29<br />
D30<br />
D31<br />
HISTOLOGIC TYPE (W HO CLASSIFICATION)** (co<br />
B lymphoblastic leukemia/lymphoma with<br />
t(1;19)(q23;p13.3); E2A-PBX1<br />
Chronic lymphocytic leukemia/small lymphocytic<br />
lymphoma<br />
B-cell prolymphocytic leukemia<br />
Splenic marginal zone lymphoma<br />
Hairy cell leukemia<br />
Splenic B-cell lymphoma/leukemia, unclassifiable<br />
Splenic diffuse red pulp small B-cell lymphoma<br />
Hairy cell leukemia-variant<br />
Lymphoplasmacytic lymphoma<br />
Waldenstrom macroglobulinemia<br />
Heavy chain disease<br />
Alpha heavy chain disease<br />
Gamma heavy chain disease<br />
Mu heavy chain disease<br />
Plasma cell myeloma<br />
Solitary plasmacytoma <strong>of</strong> bone<br />
Extraosseous plasmacytoma<br />
Extranodal marginal zone lymphoma <strong>of</strong><br />
mucosa-associated lymphoid tissue (MALT lymphoma)<br />
Extranodal marginal zone lymphoma <strong>of</strong><br />
mucosa-associated lymphoid tissue (MALT lymphoma)<br />
with plasmacytic differentiation<br />
D32<br />
D33<br />
D34<br />
D35<br />
D36<br />
D37<br />
Extranodal marginal zone lymphoma <strong>of</strong><br />
mucosa-associated lymphoid tissue (MALT lymphoma),<br />
pediatric<br />
Nodal marginal zone lymphoma<br />
Nodal marginal zone lymphoma with plasmacytic<br />
differentiation<br />
Nodal marginal zone lymphoma, pediatric<br />
Marginal zone lymphoma, not further specified<br />
Marginal zone lymphoma with plasmacytic<br />
differentiation, not further specified<br />
Follicular Lymphoma<br />
Grade<br />
D38 Follicular lymphoma, grade 1-2<br />
D39 Follicular lymphoma, grade 3A<br />
D40 Follicular lymphoma, grade 3B<br />
Growth Pattern<br />
D41 Follicular lymphoma, growth pattern - follicular<br />
D42 Follicular lymphoma, growth pattern - follicular &<br />
diffuse<br />
D43 Follicular lymphoma, growth pattern - minimally<br />
follicular<br />
D44 Follicular lymphoma, growth pattern not specified<br />
D45 Follicular lymphoma, not further specified<br />
---Histologic Types Continued on Next Page----<br />
6.2<br />
This is a worksheet. It is NOT the final diagnosis.
Non-Hodgkins Lymphoma Biopsy/Resection Synoptic Template<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
HISTOLOGIC TYPE (W HO CLASSIFICATION)** (co<br />
D63<br />
ALK positive large B-cell lymphoma<br />
D46<br />
Primary cutaneous follicle center lymphoma<br />
D64<br />
Plasmablastic lymphoma<br />
D47<br />
Primary cutaneous follicle center lymphoma vs<br />
D65<br />
Large B-cell lymphoma arising in HHV8-associated<br />
follicular lymphoma<br />
multicentric Castleman disease<br />
D48<br />
Primary cutaneous follicle center lymphoma vs diffuse<br />
D66<br />
Primary effusion lymphoma<br />
large B-cell lymphoma<br />
D67<br />
Burkitt lymphoma<br />
D49<br />
Primary cutaneous diffuse large B-cell lymphoma, leg<br />
D68<br />
B-cell lymphoma, unclassifiable, with features<br />
type vs diffuse large B-cell lymphoma, not further<br />
intermediate between diffuse large B-cell lymphoma<br />
specified<br />
and Burkitt lymphoma<br />
D50<br />
Follicular lymphoma, diffuse follicle center subtype,<br />
D69<br />
B-cell lymphoma, unclassifiable, with features<br />
grade 1-2<br />
intermediate between diffuse large B-cell lymphoma<br />
D51<br />
Mantle cell lymphoma<br />
and classical Hodgkin lymphoma<br />
D52<br />
Diffuse large B-cell lymphoma (DLBCL), NOS, germinal<br />
D70<br />
B-cell Lymphoma - Other (specify):<br />
center phenotype<br />
D53<br />
Diffuse large B-cell lymphoma (DLBCL), NOS,<br />
T-cell Lymphoma<br />
non-germinal center phenotype<br />
D71<br />
T-cell lymphoma, subtype cannot be determined<br />
D54<br />
Diffuse large B-cell lymphoma (DLBCL), NOS, not<br />
D72<br />
T-lymphoblastic leukemia/lymphoma<br />
further specified<br />
D73<br />
T-cell prolymphocytic leukemia<br />
D55<br />
T-cell/histiocyte rich large B-cell lymphoma<br />
D74<br />
T-cell large granular lymphocytic leukemia<br />
D56<br />
Primary DLBCL <strong>of</strong> the CNS<br />
D75<br />
Chronic LPD <strong>of</strong> NK-cells<br />
D57<br />
Primary Cutaneous DLBCL, leg type<br />
D76<br />
Aggressive NK-cell leukemia<br />
D58<br />
EBV positive DLBCL <strong>of</strong> the elderly<br />
D77<br />
Systemic EBV positive T-cell lymphoproliferative<br />
D59<br />
DLBCL associated with chronic inflammation<br />
disease <strong>of</strong> childhood<br />
D60<br />
Lymphomatoid granulomatosis<br />
D78<br />
Hydroa vacciniforme-like lymphoma<br />
D61<br />
D62<br />
Primary mediastinal (thymic) large B-cell lymphoma<br />
Intravascular large B-cell lymphoma<br />
D79<br />
Adult T-cell leukemia/lymphoma<br />
---Histologic Types Continued on Next Page----<br />
6.2<br />
This is a worksheet. It is NOT the final diagnosis.
Non-Hodgkins Lymphoma Biopsy/Resection Synoptic Template<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
HISTOLOGIC TYPE (W HO CLASSIFICATION)** (co<br />
ANCILLARY STUDIES**<br />
D80<br />
Extranodal NK/T-cell lymphoma, nasal type<br />
D81<br />
Enteropathy-associated T-cell lymphoma<br />
Flow Cytometry-Phenotyping**<br />
D82<br />
Hepatosplenic T-cell lymphoma<br />
E1<br />
Flow cytometry immunophenotype performed, see<br />
D83<br />
Subcutaneous panniculitis-like T-cell lymphoma<br />
separate report<br />
D84<br />
Mycosis fungoides<br />
E2<br />
Flow cytometry immunophenotype pending, report to<br />
D85<br />
Sézary syndrome<br />
follow<br />
D86<br />
Primary cutaneous anaplastic large cell lymphoma<br />
E3<br />
Flow cytometry immunophenotype not performed<br />
D87<br />
Lymphomatoid papulosis<br />
D88<br />
CD30 positive lymphoproliferative disease, NOS<br />
Immunohistochemistry/In Situ Hybridization**<br />
D89<br />
Primary cutaneous gamma-delta T-cell lymphoma<br />
F1<br />
Immunohistochemistry/ in situ hybridization performed,<br />
D90<br />
Primary cutaneous CD8-positive aggressive<br />
see microscopic description<br />
epidermotropic cytotoxic T-cell lymphoma<br />
F2<br />
Immunohistochemistry/ in situ hybridization pending,<br />
D91<br />
Primary cutaneous CD4-positive small/medium T-cell<br />
see addendum<br />
lymphoma<br />
F3<br />
Immunohistochemistry/ in situ hybridization not<br />
D92<br />
Peripheral T-cell lymphoma, NOS<br />
performed<br />
D93<br />
Angioimmunoblastic T-cell lymphoma<br />
D94<br />
Anaplastic large cell lymphoma, ALK positive<br />
Classical Cytogenetic Studies**<br />
D95<br />
Anaplastic large cell lymphoma, ALK negative (provisional)<br />
G1<br />
Classical cytogenetic studies performed, see separate<br />
D96<br />
T-cell Lymphoma - Other (specify):<br />
report<br />
G2<br />
Classical cytogenetic studies pending, report to follow<br />
Other<br />
G3<br />
Classical cytogenetic studies not performed<br />
D97<br />
Blastic plasmacytoid dendritic cell neoplasm<br />
D98<br />
Other (specify):<br />
---ANCILLARY STUDIES Continued on Next Page----<br />
6.2<br />
This is a worksheet. It is NOT the final diagnosis.
Non-Hodgkins Lymphoma Biopsy/Resection Synoptic Template<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
H1<br />
H2<br />
H3<br />
J1<br />
J2<br />
J3<br />
J4<br />
K1<br />
ANCILLARY STUDIES** (cont'd)<br />
Cytogenetic FISH Studies**<br />
Cytogenetic FISH studies performed, see separate<br />
report<br />
Cytogenetic FISH studies pending, report to follow<br />
Cytogenetic FISH studies not performed<br />
Genotypic (Molecular) Studies**<br />
Genotypic (Molecular) studies performed, see separate<br />
report<br />
Genotypic (Molecular) studies pending, report to follow<br />
Genotypic (Molecular) studies not performed<br />
DNA stored, Genotypic (Molecular) studies not<br />
performed<br />
ADDITIONAL PATHOLOGIC FINDINGS<br />
Specify:<br />
L5<br />
L6<br />
L7<br />
L8<br />
L9<br />
L10<br />
L11<br />
L12<br />
M1<br />
M2<br />
M3<br />
M4<br />
Involvement <strong>of</strong> multiple lymph node regions on both<br />
sides <strong>of</strong> diaphragm<br />
Specify sites:<br />
Splenic involvement<br />
Liver involvement<br />
Bone marrow involvement<br />
Other organ involvement<br />
Specify:<br />
Not specified<br />
CLINICAL PROGNOSTIC FACTORS AND INDICES<br />
(Select all that apply)<br />
International Prognostic Index (IPI) (specify):<br />
Follicular Lymphoma International Prognostic Index<br />
(FLIPI) (specify):<br />
B symptoms present<br />
Other (specify):<br />
L1<br />
L2<br />
L3<br />
L4<br />
EXTENT OF PATHOLOGICALLY EXAMINED TUMO<br />
(check all that apply)<br />
Involvement <strong>of</strong> a single lymph node region<br />
Specify site:<br />
Involvement <strong>of</strong> multiple lymph node regions on same<br />
side <strong>of</strong> diaphragm<br />
Specify sites:<br />
Comment:<br />
This is a worksheet. It is NOT the final diagnosis.<br />
6.2
Hodgkin Lymphoma Biopsy/Staging Synoptic Template<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
- - - - - - - - - - - - - - MACROSCOPIC - - - - - - - - - - - - - - -<br />
SPECIMEN TYPE**<br />
A1 Lymphadenectomy (specify sites):<br />
A2 Staging laparotomy<br />
A3 Extranodal (specify):<br />
A4 Other (specify):<br />
A5 Not specified<br />
PROCEDURE**<br />
B1 Fine needle aspiration<br />
B2 Needle core biopsy<br />
B3 Biopsy, other<br />
B4 Resection<br />
B5 Other (specify):<br />
B6 Unknown<br />
TUMOR SITE (check all that apply)**<br />
C1 Lymph node(s), site not specified<br />
C2 Lymph node(s) - specify sites(s):<br />
TUMOR SIZE (largest single mass) - Resections Only<br />
D1 Greatest dimensions: cm.<br />
D2 Additional dimensions: cm.<br />
D3<br />
D4<br />
E1<br />
E2<br />
E3<br />
E4<br />
E5<br />
E6<br />
E7<br />
E8<br />
Specify site:<br />
Cannot be determined (see Comment)<br />
- - - - - - - - - - - - - - MICROSCOPIC - - - - - - - - - - - - - - - -<br />
HISTOLOGIC SUBTYPE**<br />
Note: This is NOT the final diagnosis. Use final diagno<br />
/comment for therapeutic decisions.<br />
Nodular lymphocyte predominant Hodgkin lymphoma<br />
(NLPHL)<br />
Nodular sclerosis classical Hodgkin lymphoma (NSHL)<br />
Mixed cellularity classical Hodgkin lymphoma (MCHL)<br />
Lymphocyte-rich classical Hodgkin lymphoma (LRCHL)<br />
Lymphocyte-depleted classical Hodgkin lymphoma (LDHL)<br />
Classical Hodgkin lymphoma, further subtype cannot be<br />
determined<br />
Hodgkin lymphoma, subtype cannot be determined<br />
Other (specify):<br />
C3<br />
C4<br />
C5<br />
Other tissue(s) or organ(s) - specify<br />
site(s):<br />
Not specified<br />
Only one site biopsied, see above<br />
F1<br />
F2<br />
F3<br />
F4<br />
HISTOLOGIC GRADE (NSHL only) - OPTIONAL<br />
Not applicable<br />
Grade I<br />
Grade ll<br />
Grade uncertain
Hodgkin Lymphoma Biopsy/Staging Synoptic Template<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
G1<br />
G2<br />
G3<br />
G4<br />
G5<br />
G6<br />
G7<br />
G8<br />
G9<br />
H1<br />
H2<br />
H3<br />
EXTENT OF PATHOLOGICALLY EXAMINED TUMO<br />
(check all that apply)<br />
Involvement <strong>of</strong> a single lymph node region.<br />
Specify site:<br />
Involvement <strong>of</strong> multiple lymph node regions on the<br />
same side <strong>of</strong> the diaphragm.<br />
Specify sites:<br />
Involvement <strong>of</strong> multiple lymph node regions on both<br />
sides <strong>of</strong> the diaphragm.<br />
Specify sites:<br />
Splenic involvement<br />
Liver involvement<br />
Bone marrow involvement<br />
Other organ involvement<br />
Specify:<br />
Not specified<br />
ANCILLARY STUDIES**<br />
Flow Cytometry - Phenotyping**<br />
Flow cytometry immunophenotype performed, see<br />
separate report<br />
Flow cytometry immunophenotype pending, report to<br />
follow<br />
Flow cytometry immunophenotype not performed<br />
J1<br />
J2<br />
J3<br />
K1<br />
K2<br />
K3<br />
L1<br />
L2<br />
L3<br />
M1<br />
M2<br />
M3<br />
M4<br />
Immunohistochemistry/In Situ Hybridization**<br />
Immunohistochemistry/ in situ hybridization performed,<br />
see microscopic description<br />
Immunohistochemistry/ in situ hybridization pending,<br />
see addendum<br />
Immunohistochemistry/ in situ hybridization not<br />
performed<br />
Classical Cytogenetic Studies**<br />
Classical cytogenetic studies performed, see separate<br />
report<br />
Classical cytogenetic studies pending, report to follow<br />
Classical cytogenetic studies not performed<br />
Cytogenetic FISH Studies**<br />
Cytogenetic FISH studies performed, see separate<br />
report<br />
Cytogenetic FISH studies pending, report to follow<br />
Cytogenetic FISH studies not performed<br />
Genotypic (Molecular) Studies**<br />
Genotypic (Molecular) studies performed, see separate<br />
report<br />
Genotypic (Molecular) studiending, report to follow<br />
Genotypic (Molecular) studies not performed<br />
DNA stored,Genotypic (Molecular) studies not performed
Hodgkin Lymphoma Biopsy/Staging Synoptic Template<br />
Specimen #:<br />
Patient:<br />
Part:<br />
Initials/Date:<br />
ADDITIONAL PATHOLOGIC FINDINGS<br />
(check all that apply)<br />
N1 Progressive transformation <strong>of</strong> germinal centers (PTGC)<br />
N2 Castleman disease<br />
N3 Other (specify): ________________________________<br />
CLINICAL PROGNOSTIC FACTORS AND INDICES<br />
P1 International Prognostic Score (IPS) (specify):<br />
P2<br />
P3<br />
B symptoms present<br />
Other (specify): ____________<br />
Comment:
Web-Based Resources
WEBSITE EDUCATIONAL RESOURCES & CALL SCHEDULES<br />
Division <strong>of</strong> <strong>Hematopathology</strong> Intranet website (<strong>Hematopathology</strong> schedules/paging and general<br />
information)<br />
1. http://path.upmc.edu/divisions/hematopath.html<br />
UPMC Pathology <strong>Resident</strong>s Server (includes on call schedule for AP and CP)<br />
1. http://residents.pathology.pitt.edu/<br />
Hematological Malignancies Program<br />
1. http://www.upmccancercenter.com/portal_hema/overview.cfm<br />
For general pathology (<strong>Hematopathology</strong>, Dermatopathology and others)<br />
1. http://www-medlib.med.utah.edu/WebPath/webpath.html<br />
2. http://dermatlas.med.jhmi.edu/derm (Users can search by categories, diagnoses, or body site)<br />
3. www.uscap.org (numerous teaching resources)<br />
4. http://www.pathologyoutlines.com<br />
HEMATOPATHOLOGY<br />
Atlas<br />
1. http://www.ashimagebank.org/<br />
2. http://www.bloodmed.com/home/<br />
Virtual Slide Set<br />
1. https://secure.opi.upmc.edu/hematopathology/index.cfm (or link via residents web page<br />
http://residents.pathology.pitt.edu/ -- the link to <strong>Hematopathology</strong> virtual slide set is on the right hand<br />
side (direct link to virtual slide set Division <strong>of</strong> <strong>Hematopathology</strong> UPMC Presbyterian)<br />
2. http://cclcm.ccf.org/vm/VM_cases/lymphoid_main.htm<br />
(Virtual <strong>Hematopathology</strong> slides (and other fixed images) from Cleveland Clinic)<br />
3. www.uscap.org (Virtual Slide Box at USCAP)<br />
General<br />
1. http://www.sh-eahp.org/<br />
2. http://medmark.org/hem/<br />
3. http://www.cancer.gov/ (treatment <strong>of</strong> leukemias/lymphoma)<br />
Case Studies<br />
1. http://path.upmc.edu/cases.html<br />
2. http://teachingcases.hematology.org/<br />
USCAP <strong>Hematopathology</strong> Evening Session Cases<br />
1. http://www.uscap.org/<br />
Societies<br />
1. http://www.hematology.org/<br />
2. http://www.aspho.org/<br />
3. http://www.blacksci.co.uk/uk/society/bsh/default.htm<br />
4. http://www.leukemia.org/
Educational Site<br />
1. http://www.cyto.purdue.edu/index.htm<br />
2. http://flowcyt.cyto.purdue.edu/flowcyt/educate/pptslide.htm<br />
Cases and Tests<br />
1. http://www.madsci.org/~lynn/VH/APIII/CPflow.html<br />
Societies<br />
1. http://www.cytometry.org/<br />
2. http://www.isac-net.org/<br />
Books<br />
1. http://www.cyto.purdue.edu/cdroms/cyto2/14/pucl/flowcyt/refclin.htm<br />
CYTOGENETICS<br />
1. http://www.pathology.washington.edu/Cytogallery/<br />
2. http://cgap.nci.nih.gov/cgap.html<br />
NOTE: IF THERE ARE ANY SITES YOU FEEL SHOULD BE ADDED TO THIS LIST OR DEFUNCT SITES,<br />
PLEASE LET DR. SWERDLOW OR DR. ROTH KNOW<br />
Online Conference Access for Pathology Faculty and <strong>Resident</strong>s<br />
• Visit <strong>Department</strong> <strong>of</strong> Pathology Conference Access for Pathology Faculty and <strong>Resident</strong>s<br />
• http://teleconference.upmc.edu/index.html<br />
Weekly Conference includes:<br />
• Anatomic Pathology Grand Rounds conference<br />
• <strong>Department</strong>al Seminar (Archived)<br />
These LIVE and archived conferences are only accessible through the UPMC network.<br />
Note: Additional non-web based resources are in UPMC-<br />
Presbyterian G304, G315 and G323.