SHOT Annual Report 2009 - Serious Hazards of Transfusion
SHOT Annual Report 2009 - Serious Hazards of Transfusion
SHOT Annual Report 2009 - Serious Hazards of Transfusion
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
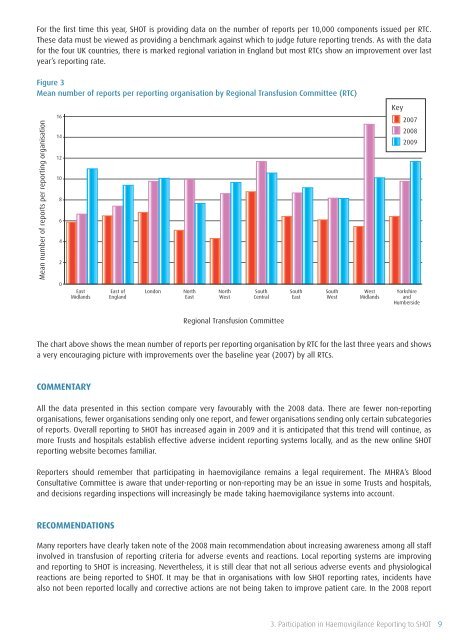
For the first time this year, <strong>SHOT</strong> is providing data on the number <strong>of</strong> reports per 10,000 components issued per RTC.<br />
These data must be viewed as providing a benchmark against which to judge future reporting trends. As with the data<br />
for the four UK countries, there is marked regional variation in England but most RTCs show an improvement over last<br />
year’s reporting rate.<br />
Figure 3<br />
Mean number <strong>of</strong> reports per reporting organisation by Regional <strong>Transfusion</strong> Committee (RTC)<br />
Mean number <strong>of</strong> reports per reporting organisation<br />
16<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
Key<br />
2007<br />
2008<br />
<strong>2009</strong><br />
0<br />
East<br />
Midlands<br />
East <strong>of</strong><br />
England<br />
London<br />
North<br />
East<br />
North<br />
West<br />
South<br />
Central<br />
South<br />
East<br />
South<br />
West<br />
West<br />
Midlands<br />
Yorkshire<br />
and<br />
Humberside<br />
Regional <strong>Transfusion</strong> Committee<br />
The chart above shows the mean number <strong>of</strong> reports per reporting organisation by RTC for the last three years and shows<br />
a very encouraging picture with improvements over the baseline year (2007) by all RTCs.<br />
COMMENTARY<br />
All the data presented in this section compare very favourably with the 2008 data. There are fewer non-reporting<br />
organisations, fewer organisations sending only one report, and fewer organisations sending only certain subcategories<br />
<strong>of</strong> reports. Overall reporting to <strong>SHOT</strong> has increased again in <strong>2009</strong> and it is anticipated that this trend will continue, as<br />
more Trusts and hospitals establish effective adverse incident reporting systems locally, and as the new online <strong>SHOT</strong><br />
reporting website becomes familiar.<br />
<strong>Report</strong>ers should remember that participating in haemovigilance remains a legal requirement. The MHRA’s Blood<br />
Consultative Committee is aware that under-reporting or non-reporting may be an issue in some Trusts and hospitals,<br />
and decisions regarding inspections will increasingly be made taking haemovigilance systems into account.<br />
RECOMMENDATIONS<br />
Many reporters have clearly taken note <strong>of</strong> the 2008 main recommendation about increasing awareness among all staff<br />
involved in transfusion <strong>of</strong> reporting criteria for adverse events and reactions. Local reporting systems are improving<br />
and reporting to <strong>SHOT</strong> is increasing. Nevertheless, it is still clear that not all serious adverse events and physiological<br />
reactions are being reported to <strong>SHOT</strong>. It may be that in organisations with low <strong>SHOT</strong> reporting rates, incidents have<br />
also not been reported locally and corrective actions are not being taken to improve patient care. In the 2008 report<br />
3. Participation in Haemovigilance <strong>Report</strong>ing to <strong>SHOT</strong><br />
9