Calcium Phosphate Excipients - Innophos
Calcium Phosphate Excipients - Innophos
Calcium Phosphate Excipients - Innophos
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Relative A.N.C. (%)<br />
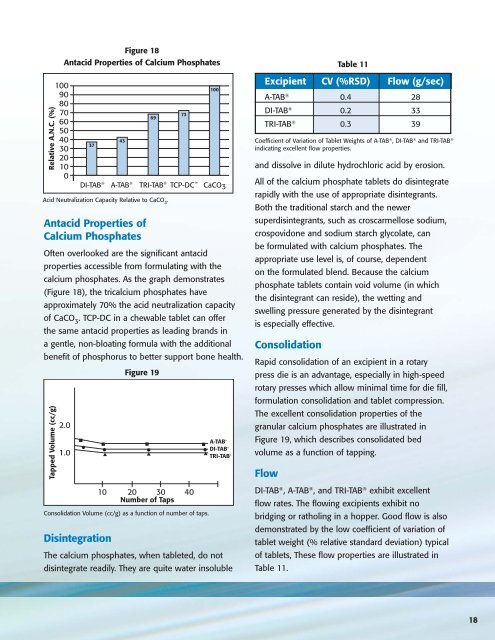
Figure 18<br />
Antacid Properties of <strong>Calcium</strong> <strong>Phosphate</strong>s<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
37<br />
DI-TAB ®<br />
Disintegration<br />
43<br />
A-TAB ®<br />
Acid Neutralization Capacity Relative to CaCO 3 .<br />
Antacid Properties of<br />
<strong>Calcium</strong> <strong>Phosphate</strong>s<br />
TRI-TAB ® TCP-DC <br />
The calcium phosphates, when tableted, do not<br />
disintegrate readily. They are quite water insoluble<br />
69<br />
73<br />
100<br />
CaCO3<br />
Often overlooked are the significant antacid<br />
properties accessible from formulating with the<br />
calcium phosphates. As the graph demonstrates<br />
(Figure 18), the tricalcium phosphates have<br />
approximately 70% the acid neutralization capacity<br />
of CaCO 3<br />
. TCP-DC in a chewable tablet can offer<br />
the same antacid properties as leading brands in<br />
a gentle, non-bloating formula with the additional<br />
benefit of phosphorus to better support bone health.<br />
Tapped Volume (cc/g)<br />
2.0<br />
1.0<br />
Figure 19<br />
10 20 30 40<br />
Number of Taps<br />
Consolidation Volume (cc/g) as a function of number of taps.<br />
A-TAB ®<br />
DI-TAB ®<br />
TRI-TAB ®<br />
Excipient CV (%RSD) Flow (g/sec)<br />
A-TAB ® 0.4 28<br />
DI-TAB ® 0.2 33<br />
TRI-TAB ® 0.3 39<br />
and dissolve in dilute hydrochloric acid by erosion.<br />
All of the calcium phosphate tablets do disintegrate<br />
rapidly with the use of appropriate disintegrants.<br />
Both the traditional starch and the newer<br />
superdisintegrants, such as croscarmellose sodium,<br />
crospovidone and sodium starch glycolate, can<br />
be formulated with calcium phosphates. The<br />
appropriate use level is, of course, dependent<br />
on the formulated blend. Because the calcium<br />
phosphate tablets contain void volume (in which<br />
the disintegrant can reside), the wetting and<br />
swelling pressure generated by the disintegrant<br />
is especially effective.<br />
Consolidation<br />
Rapid consolidation of an excipient in a rotary<br />
press die is an advantage, especially in high-speed<br />
rotary presses which allow minimal time for die fill,<br />
formulation consolidation and tablet compression.<br />
The excellent consolidation properties of the<br />
granular calcium phosphates are illustrated in<br />
Figure 19, which describes consolidated bed<br />
volume as a function of tapping.<br />
Flow<br />
Table 11<br />
Coefficient of Variation of Tablet Weights of A-TAB ® , DI-TAB ® and TRI-TAB ®<br />
indicating excellent flow properties.<br />
DI-TAB ® , A-TAB ® , and TRI-TAB ® exhibit excellent<br />
flow rates. The flowing excipients exhibit no<br />
bridging or ratholing in a hopper. Good flow is also<br />
demonstrated by the low coefficient of variation of<br />
tablet weight (% relative standard deviation) typical<br />
of tablets, These flow properties are illustrated in<br />
Table 11.<br />
18