Key Concept Chart - Pearson
Key Concept Chart - Pearson
Key Concept Chart - Pearson
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
‘<br />
Integrated Science<br />
<strong>Key</strong> <strong>Concept</strong> <strong>Chart</strong><br />
Form 3<br />
Science, technology and society<br />
Useful definitions<br />
anti-virus software: software that has been written to find and<br />
destroy viruses<br />
desktop publishing (DTP): the process of creating complex<br />
documents such as magazines on a computer<br />
download: a piece of software obtained from a server on the<br />
Internet and transferred onto your computer<br />
email: messages sent from one computer user to another using<br />
electronic mailboxes on email servers on the Internet<br />
hardware: the electronics of a computer<br />
Internet service provider: a company that sells the use of their<br />
email and web servers to the public<br />
online: connected to the Internet<br />
portable document format (PDF): a small software document<br />
(small number of bytes) that can easily be sent by email<br />
search engine: software on a server that allows users to search for<br />
anything on the World Wide Web<br />
viruses: pieces of computer software that can spread on the<br />
Internet and damage computers<br />
web browsers: software in a computer that allows users to find<br />
and read websites<br />
web server: a computer that contains websites that users can read<br />
on the World Wide Web<br />
word-processing: the commonly used process of creating<br />
electronic documents such as letters on a computer<br />
The nature of matter<br />
Useful definitions<br />
atom: the basic building block of matter; the smallest particle that<br />
all matter is made of<br />
atomic number: the number of protons in an atom<br />
boiling point: the temperature at which a liquid boils<br />
compound: a chemical combination of two or more elements in<br />
fixed proportions<br />
diatomic molecules: molecules that are made up of two atoms<br />
distillation: a purification process that uses the difference in<br />
boiling points of the constituents of a mixture<br />
electron configuration: the arrangement of the electrons in an<br />
atom or molecule<br />
element: a substance that contains only one type of atom<br />
evaporation: the process by which some materials change from<br />
liquid to gas<br />
isotopes: atoms of the same element with different masses<br />
mass number: the total number of protons and neutrons in the<br />
nucleus of an atom<br />
matter: all objects in the universe are made of matter<br />
melting point: the temperature at which a substance changes<br />
from solid to liquid<br />
mixture: a combination of elements in any proportion that can be<br />
separated by physical processes<br />
molecule: two or more atoms joined together<br />
nucleus: the centre of an atom containing the protons and neutrons<br />
orbit: to move around something<br />
orbitals: the different energy levels around the nucleus that<br />
electrons occupy<br />
reactivity: the rate or speed at which a chemical substance tends<br />
to undergo a chemical reaction<br />
Managing natural resources<br />
• Pollution results when any form of harmful substance enters<br />
the environment.<br />
• The three main types of pollution are air, water and soil<br />
pollution.<br />
• Examples of sources of pollution are: burning of fossil fuels,<br />
poisons, sewage, plastics, pesticides, toxic waste, mines,<br />
agriculture, power plants and construction sites.<br />
• Problems caused by pollution include: acid rain, global<br />
warming, polluted water, contaminated soil and health<br />
problems (lung diseases, cholera, dysentery, typhoid<br />
and cancer).<br />
• Ways in which we can manage pollution include: educating<br />
people about its dangers, handling and disposing of waste<br />
correctly, reducing the amount of pollutants produced, using<br />
environmentally-friendly products, and reducing the use of<br />
fossil fuels.<br />
Acids and bases<br />
• pH tells us how acidic or basic a substance is. It is measured<br />
on a scale from 0 to 14.<br />
• The strength of a substance relates to how well it dissociates<br />
when dissolved in water, while the concentration is a<br />
measure of how much of a substance is dissolved.<br />
• A strong acid has a low pH.<br />
• A weak acid has a high pH.<br />
• A concentrated acid has a lot of acid and a little water.<br />
• A diluted acid has a lot of water and a little acid.<br />
When a metal reacts with an acid:<br />
acid + metal → salt + hydrogen gas<br />
When a carbonate reacts with an acid:<br />
carbonate + acid → salt + carbon dioxide + water<br />
The pH Scale:<br />
in Action integrated science in Action integrated science in Action integrated science in Action integrated science
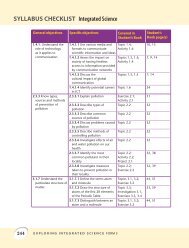
Periodic Table<br />
}<br />
7<br />
Group 1 elements<br />
These are soft light metals. They react very quickly<br />
to water, are similar and are called alkali metals.<br />
11<br />
I<br />
Li<br />
Lithium<br />
Na<br />
Sodium<br />
9<br />
II<br />
Be<br />
Berryllium<br />
12<br />
Mg<br />
Magnesium<br />
1<br />
H<br />
Hydrogen<br />
Transition elements<br />
These are unreactive metals. They have very high melting points.<br />
They are very useful. Many of their compounds are coloured.<br />
Group VIII elements<br />
(called group 0)<br />
These are all gases. They do not form compounds.<br />
They are called noble or inert gases.<br />
Group VII elements<br />
These are very reactive non-metals.<br />
They are poisonous, but some of their<br />
compounds (like table salt) are essential<br />
for us to live. They are called halogens.<br />
5<br />
13<br />
III IV V VI VII<br />
B<br />
Boron<br />
Ai<br />
Aluminium<br />
6<br />
14<br />
C<br />
Carbon<br />
Si<br />
Silicon<br />
7<br />
15<br />
N<br />
Nitrogen<br />
P<br />
Phosphorous<br />
8<br />
16<br />
O<br />
Oxygen<br />
S<br />
Sulphur<br />
}<br />
9<br />
17<br />
F<br />
Fluorine<br />
Cl<br />
Chlorine<br />
}<br />
VIII<br />
2<br />
He<br />
10<br />
Helium<br />
Ne<br />
18<br />
Neon<br />
Ar<br />
Argon<br />
19<br />
K<br />
20<br />
Ca<br />
21<br />
Sc<br />
22<br />
Ti<br />
23<br />
V<br />
24<br />
Cr<br />
25 26 27 28<br />
Mn<br />
Fe<br />
Co<br />
Ni<br />
29<br />
Cu<br />
30<br />
Zn<br />
31 32 33 34<br />
Ga<br />
Ge<br />
As<br />
Se<br />
35<br />
Br<br />
36<br />
Kr<br />
Potassium<br />
Calcium<br />
Scandium<br />
Titanium<br />
Vanadium<br />
Chromium<br />
Manganese<br />
Iron<br />
Cobalt<br />
Nickel<br />
Copper<br />
Zinc<br />
Gallium<br />
Germanium<br />
Arsenic<br />
Selenium<br />
Bromine<br />
Krypton<br />
37<br />
Rb<br />
38<br />
Sr<br />
39<br />
Y<br />
40<br />
Zr<br />
41<br />
Nb<br />
42<br />
Mo<br />
43<br />
Tc<br />
44 45 46 47<br />
Ru<br />
Rh<br />
Pd<br />
Ag<br />
48<br />
Cd<br />
49<br />
In<br />
50 51 52 53<br />
Sn<br />
Sb<br />
Te<br />
I<br />
54<br />
Xe<br />
Rubidium<br />
Strontium<br />
Yttrium<br />
Zirconium<br />
Niobium<br />
Molybdenum<br />
Technetium<br />
Ruthenium<br />
Rhodium<br />
Palladium<br />
Silver<br />
Cadmium<br />
Indium<br />
Tin<br />
Antimony<br />
Tellurium<br />
Iodine<br />
Xenon<br />
55<br />
Cs<br />
56<br />
Ba<br />
57<br />
La<br />
72<br />
Hf<br />
73<br />
Ta<br />
74<br />
W<br />
75<br />
Re<br />
76 77 78 79<br />
Os<br />
Ir<br />
Pt<br />
Au<br />
80<br />
Hg<br />
81<br />
Tl<br />
82 83 84 85<br />
Pb<br />
Bi<br />
Po<br />
At<br />
86<br />
Rn<br />
Cesium<br />
Barium<br />
Lanthamium<br />
Hafnium<br />
Tantalum<br />
Tungsten<br />
Rhenium<br />
Osmium<br />
Iridium<br />
Platinum<br />
Gold<br />
Mercury<br />
Thallium<br />
Lead<br />
Bismuth<br />
Polonium<br />
Astatine<br />
Radon<br />
87<br />
Fr<br />
88<br />
Ra<br />
89<br />
Ac<br />
104<br />
Rf<br />
105<br />
Db<br />
106<br />
Sg<br />
107<br />
Bh<br />
108<br />
Hs<br />
109<br />
Mt<br />
Francium<br />
Radium<br />
Actinium<br />
Rutherfordium<br />
Dubnium<br />
Seaborgium<br />
Bohrium<br />
Hassium<br />
Meitnerium<br />
58<br />
Ce<br />
59<br />
Pr<br />
60<br />
Nd<br />
61 62 63 64<br />
Pm<br />
Sm<br />
Eu<br />
Gd<br />
65<br />
Tb<br />
66<br />
Dy<br />
67 68 69 70<br />
Ho<br />
Er<br />
Tm<br />
Yb<br />
71<br />
Lu<br />
Cerium<br />
Praseodymium<br />
Neodymium<br />
Promethium<br />
Samarium<br />
Europium<br />
Gadolinium<br />
Terbium<br />
Dysprosium<br />
Holmium<br />
Erbium<br />
Thulium<br />
Ytterbium<br />
Lutetium<br />
90<br />
Th<br />
91<br />
Pa<br />
92<br />
U<br />
93 94 95 96<br />
Np<br />
Pu<br />
Am<br />
Cm<br />
97<br />
Bk<br />
98<br />
Cf<br />
99<br />
Es<br />
100<br />
Fm<br />
101<br />
Md<br />
102<br />
No<br />
103<br />
Lr<br />
Thorium<br />
Protactimium<br />
Uranium<br />
Neptunium<br />
Plutonium<br />
Americium<br />
Curium<br />
Berkelium<br />
Californium<br />
Einsteinium<br />
Fermium<br />
Mendelevium<br />
Nobelium<br />
Lawrencium<br />
KEY<br />
Reactive<br />
metals<br />
Transition<br />
metals<br />
Less reactive<br />
metals<br />
Non-metals<br />
Noble gases<br />
Zig-zag line<br />
separates the metals<br />
from the non-metals<br />
1<br />
H<br />
Hydrogen<br />
atomic<br />
number<br />
symbol<br />
name<br />
in Action integrated science in Action integrated science in Action integrated science in Action integrated science
Metals and non-metals<br />
Most metals react with oxygen to form metal oxides.<br />
This process is called corrosion.<br />
Metals are mixed with other metals (and sometimes<br />
with non-metals) to make substances called alloys.<br />
Alloys have properties that the original metals do<br />
not have. Brass, bronze and solder are examples<br />
of alloys.<br />
Carbon is a common non-metallic element found<br />
in all molecules of living things. Carbon can exist<br />
in more than one form in the solid state. Different<br />
forms of the same element in the same state<br />
are known as allotropes. Carbon allotropes are<br />
diamonds, graphite and fullerene.<br />
Machines<br />
• Simple machines make work easier.<br />
• A lever is a simple machine.<br />
• You apply a small force at the effort to create a big<br />
force at the load.<br />
• A lever is supported at the fulcrum or pivot.<br />
• An inclined plane enables you to lift objects more<br />
easily and with a smaller force than if you lifted<br />
them vertically.<br />
• The moment of force is the product of the force<br />
applied at a point, and the perpendicular distance<br />
from where the force is acting (e.g. opening a door).<br />
• The Principle of Moments states that when a<br />
beam is balanced, the clockwise motion is equal<br />
to the anticlockwise moment.<br />
Physical and chemical properties of metals<br />
Physical properties<br />
Conduct heat well<br />
Good electrical conductors<br />
Surface is shiny when<br />
clean – this is called lustre<br />
Malleable – they can be<br />
rolled into thin sheets<br />
Ductile – they can be<br />
pulled into thin wires<br />
Sonorous – they make a<br />
sound when hit<br />
Usually solid at room<br />
temperature (except<br />
mercury)<br />
Chemical properties<br />
Usually have 1–3 electrons<br />
in their outer shell<br />
Lose the electrons in their<br />
outer shell easily<br />
React with oxygen to<br />
produce basic oxides<br />
Good reducing agents<br />
Physical and chemical properties of non-metals<br />
Physical properties<br />
Poor conductors of heat –<br />
they are insulators<br />
Chemical properties<br />
Usually have 4–8 electrons<br />
in their outer shell<br />
load (large force)<br />
Electricity and magnetism<br />
• A resistor is any conductor that resists the flow of<br />
current in a circuit. Bulbs are resistors.<br />
• We measure resistance in units called ohms Ω.<br />
• In a resistor, electrical energy is changed into<br />
other forms of energy such as heat and light.<br />
• This voltage drop across a resistor is a measure of<br />
how much energy is converted to heat and light<br />
energy in the resistor.<br />
• The current passing through a resistor is directly<br />
proportional to the potential difference across it.<br />
• The resistance of a wire increases when it gets hot.<br />
Remember:<br />
fulcrum<br />
A spade being used as a lever<br />
effort (small force)<br />
Do not conduct electricity<br />
well<br />
Brittle if they are solids<br />
Not ductile<br />
Do not have a metallic<br />
lustre<br />
Can be solids, liquids or<br />
gases at room temperature<br />
Gain or share the electrons<br />
in their outer shell easily<br />
React with oxygen to form<br />
oxides that are acidic<br />
Good oxidising agents<br />
R = V ; V = IR; I = V<br />
I<br />
R<br />
Resistance = voltage<br />
current<br />
If several resistors are connected in series in a circuit:<br />
R = R1 + R2 + R3 + …<br />
If several resistors are connected in parallel in a<br />
circuit: 1 1 1<br />
= + + …<br />
R R1 R1<br />
in Action integrated science in Action integrated science in Action integrated science in Action integrated science
This new series consists of student’s books, teacher’s guides,<br />
key concept charts and test CDs, all aligned to the Revised<br />
Junior Secondary School curriculum for Botswana.<br />
IA INT SCIENCE 3SB CV.indd 1<br />
This course is supported by the online<br />
Your complete classroom solution!<br />
www.longmanafrica.co.za<br />
Longman books are printed on quality paper,<br />
and have sturdy, long-lasting covers.<br />
ISBN 978-99912-595-8-1<br />
9 789991 259581<br />
NEW<br />
CURRICULUM<br />
with Student’s Book!<br />
with Teacher’s<br />
Guide!<br />
Janice Barrett | Alpa Somaiya<br />
Reviewed by: Masego Basimanebotlhe<br />
2011/07/14 03:48:44 PM<br />
TOPIC 9 Body systems<br />
In Form 2, you learnt some important things about the<br />
human body. For example, you studied how your body is used<br />
for communication and<br />
The<br />
how<br />
human<br />
the sense<br />
body<br />
organs help you to<br />
communicate. You also learnt that the nervous system is made up<br />
of • many The nerves functions that make of the different skeleton muscles are react. to protect Some of these<br />
muscles, together with bones and joints, enable you to move your<br />
internal organs, support the muscles and body<br />
body. For example, you use muscles, bones and joints to walk, hold<br />
a pencil,<br />
organs<br />
kick<br />
and<br />
a soccer<br />
allow<br />
ball,<br />
body<br />
and so<br />
movement.<br />
on.<br />
• A joint is a part of the body that can bend<br />
In this because unit, we two will learn bones about meet the there. physiology of bones, muscles<br />
and • joints. For movement to happen, the action of the<br />
The muscles, main functions bones of and the joints human must skeleton be coordinated.<br />
The adult<br />
Muscles<br />
human<br />
are<br />
skeleton<br />
attached<br />
is made<br />
to bones<br />
up of 206<br />
by<br />
different<br />
tendons.<br />
bones.<br />
Limbs<br />
These<br />
bones function begin to develop as levers. before Bones birth. are As pulled newborn by babies, pairs our of bones<br />
are soft, muscles and our to bodies move are the floppy. levers. As we get older, our bones get<br />
hard • Posture and strong is and the we position can or of stand the body up straight. parts relative<br />
to one another.<br />
Look at Figure 9.1 to see all the different bones in the adult<br />
skeleton.<br />
• Good<br />
Notice<br />
posture<br />
that most<br />
involves<br />
of the<br />
aligning<br />
bones have<br />
each<br />
scientific<br />
part of<br />
names, for<br />
example, the body humerus with and the femur. neighbouring You do not need parts, to remember thereby the<br />
names keeping of the bones! them well-balanced and supported.<br />
skull<br />
cervical vertebrae<br />
clavicle<br />
scapula<br />
sternum<br />
humerus<br />
ribs<br />
vertebral column<br />
pelvis<br />
radius<br />
ulna<br />
carpals<br />
metatarsals<br />
phalanges<br />
femur<br />
patella<br />
tibia<br />
fibula<br />
The human skeleton<br />
HIV/AIDS<br />
AIDS care-givers must:<br />
• provide emotional support and practical help to<br />
AIDS patients,<br />
• respect the confidentiality of AIDS patients,<br />
• know how to prevent infections, control pain and<br />
cope with very ill patients, and<br />
• encourage patients to do gentle exercise, eat<br />
healthily and take sufficient rest.<br />
Force, motion and energy<br />
• Forces can cause stationary objects to move and<br />
moving objects to change direction or slow down.<br />
• Newton’s First Law of Motion states that an object<br />
remains at rest, or if it is moving it will continue to<br />
move with constant speed in the same direction,<br />
until a force acts on it to move it differently.<br />
• Newton’s Second Law of Motion says that when<br />
a force is applied to a moving object it causes the<br />
momentum of the object to change. The rate of<br />
change of this momentum is equal to the size of<br />
the force. The change takes place in the direction<br />
of the force.<br />
• A useful way of expressing Newton’s Second<br />
Law is the formula F = ma, where F is the force<br />
applied, m is the mass of the object and a is the<br />
acceleration of the object caused by the force.<br />
• Newton’s Third Law of Motion says that action<br />
and reaction are equal and opposite. This means<br />
that if you push against a wall, the wall is also<br />
pushing against you with the same force.<br />
New word<br />
physiology the study of<br />
how living bodies work<br />
<strong>Key</strong> concept<br />
The human skeleton is<br />
made up of 206 different<br />
bones. The main functions<br />
of the human skeleton are:<br />
to give sturdiness and<br />
provide a frame, to provide<br />
attachments for muscles<br />
and ligaments, to enable<br />
the body to move, and to<br />
provide protection for vital<br />
organs.<br />
Emerging issue<br />
Children must have good<br />
nutrition, because bones<br />
need calcium, and muscles<br />
need carbohydrates and<br />
protein to become strong<br />
and healthy.<br />
The solar system<br />
Space exploration:<br />
• provides us with more information about space<br />
beyond our Earth,<br />
• tells us what the universe was like in the distant past,<br />
• is expensive and dangerous,<br />
• has contributed to advances in types of plastic,<br />
television, computers and human health, and<br />
• relies on the skills of astronauts, pilots, computer<br />
technicians, mechanics, medical doctors, scientists,<br />
engineers, chemists and geologists.<br />
Satellites give us information about Earth. Some<br />
research satellites contain devices that can study<br />
the universe 199 from above the atmosphere, obtaining<br />
information that we cannot get on Earth.<br />
Space probes send information back about other<br />
parts of the solar system. There are many different<br />
kinds of telescopes that detect radio waves, X-rays,<br />
heat and light from objects in the universe.<br />
The solar system<br />
Figure 9.1 A front and back<br />
view of the human skeleton<br />
SO 7.4.1.1, 7.4.1.2<br />
Sun<br />
Mercury<br />
Mars<br />
Earth<br />
Saturn<br />
Uranus<br />
LONGMAN<br />
for success in Form 3!<br />
• Comprehensive content coverage<br />
• Easy, student-friendly explanations<br />
• Local examples<br />
• Exam-style assessments<br />
LONGMAN<br />
your complete classroom solution!<br />
Contact details<br />
<strong>Pearson</strong> Botswana: Tel: +267 3922969 Fax: +267 3922682<br />
Plot 14386, New Lobatse Road, G-West Industrial Site,<br />
LONGMAN<br />
Gaborone, Botswana. Website: www.longmanafrica.co.za<br />
Integrated Science Form 3<br />
FREE<br />
KEY CONCEPT CHART<br />
FREE<br />
TEST CD<br />
Form 3 • Student’s Book<br />
Venus<br />
Jupiter<br />
Neptune<br />
ISBN 978- 99912-580-5- 8<br />
9 789991 258058