A natron source at Pikrolimni Lake in Greece? Geochemical evidence
A natron source at Pikrolimni Lake in Greece? Geochemical evidence
A natron source at Pikrolimni Lake in Greece? Geochemical evidence
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
142 E. Dotsika et al. / Journal of <strong>Geochemical</strong> Explor<strong>at</strong>ion 103 (2009) 133–143<br />
Table 3<br />
Thermodynamic calcul<strong>at</strong>ion model: sequence of m<strong>in</strong>erals precipit<strong>at</strong>ion.<br />
<strong>Lake</strong> <strong>Pikrolimni</strong> 8/2002 <strong>Lake</strong> <strong>Pikrolimni</strong> 9/2006 <strong>Lake</strong> Magadi, Kenya a <strong>Lake</strong> N<strong>at</strong>ron, Tanzania a The Deep Spr<strong>in</strong>gs <strong>Lake</strong>, California a<br />
Calcite (CaCO 3 )<br />
Dolomite [Ca(Mg)CO 3 )]<br />
Calcite (CaCO 3 )<br />
Dolomite [Ca(Mg)CO 3 )]<br />
Calcite (CaCO 3 ) Calcite–Mg [Ca(Mg)CO 3 ]<br />
Dolomite (MgCO 3 )<br />
Gaylussite [Na 2 Ca⁎<br />
(CO 3 ) 2 ⁎5H 2 O]<br />
Gaylussite [Na 2 Ca⁎<br />
(CO 3 ) 2 ⁎5H 2 O]<br />
Gaylussite [Na 2 Ca⁎<br />
(CO 3 ) 2 ⁎5H 2 O]<br />
Pirssonite [Na 2 Ca⁎<br />
(CO 3 ) 2 ⁎2H 2 O]<br />
Pirssonite [Na 2 Ca⁎<br />
(CO 3 ) 2 ⁎2H 2 O]<br />
Pirssonite [Na 2 Ca⁎<br />
(CO 3 ) 2 ⁎2H 2 O]<br />
Nahcolite (NaHCO 3 ) Nacholite (NaHCO 3 ) Nahcolite (NaHCO 3 ) Nahcolite (NaHCO 3 ) Nahcolite (NaHCO 3 )<br />
N<strong>at</strong>ron (Na 2 CO 3 ⁎10H 2 O) N<strong>at</strong>ron (Na 2 CO 3 ⁎10H 2 O)<br />
Trona (Na 2 CO 3 ⁎NaHCO 3 ⁎2H 2 O Trona (Na 2 CO 3 ⁎NaHCO 3 ⁎2H 2 O Trona (Na 2 CO 3 ⁎NaHCO 3 ⁎2H 2 O) Trona (Na 2 CO 3 ⁎NaHCO 3 ⁎2H 2 O Trona (Na 2 CO 3 ⁎NaHCO 3 ⁎2H 2 O<br />
Thermon<strong>at</strong>rite (Na 2 CO 3 ⁎H 2 O) Thermon<strong>at</strong>rite (Na 2 CO 3 ⁎H 2 O)<br />
Gypsum (CaSO 4 ⁎2H 2 O)<br />
Glauberite [Na 2 Ca(SO 4 )]<br />
Burkeite (Na 2 CO 3 ⁎2Na 2 SO 4 )<br />
Mirabilite (Na 2 SO 4 ⁎10H 2 O) Mirabilite (Na 2 SO 4 ⁎10H 2 O)<br />
Thenardite (Na 2 SO 4 ) Thenardite (Na 2 SO 4 )) Thenardite (Na 2 SO 4 )<br />
Halite (NaCl) Halite (NaCl) Halite (NaCl) Halite (NaCl) Halite (NaCl)<br />
a Gueddari M. (1984).<br />
Table 4<br />
Simul<strong>at</strong>ion of evapor<strong>at</strong>ion of groundw<strong>at</strong>er.<br />
Label<br />
S/1_/2002<br />
b/2/2002<br />
b/3/2002<br />
b/4/2002<br />
b/A/2002<br />
wt/B/2002<br />
b/G/2003<br />
b/G/2004<br />
b/P1/7/2004<br />
b/G/7/2004<br />
b/B/8/2004<br />
b/SPA<br />
wt/SPA<br />
MWP-1<br />
MWPh-1<br />
Pl-5/2003<br />
M<strong>in</strong>erals<br />
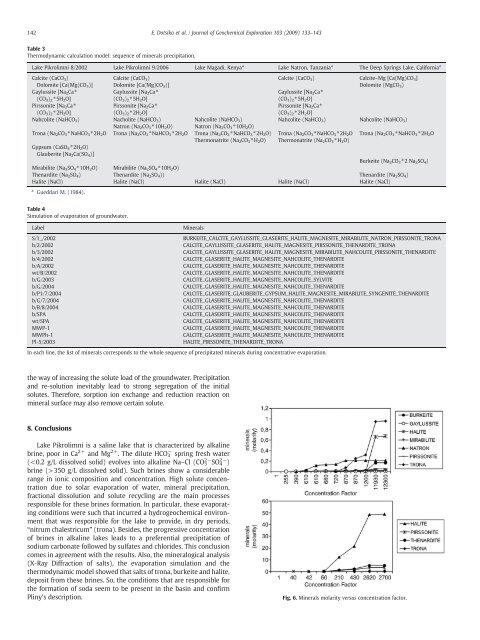
BURKEITE_CALCITE_GAYLUSSITE_GLASERITE_HALITE_MAGNESITE_MIRABILITE_NATRON_PIRSSONITE_TRONA<br />
CALCITE_GAYLUSSITE_GLASERITE_HALITE_MAGNESITE_PIRSSONITE_THENARDITE_TRONA<br />
CALCITE_GAYLUSSITE_GLASERITE_HALITE_MAGNESITE_MIRABILITE_NAHCOLITE_PIRSSONITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_SYLVITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_GLAUBERITE_GYPSUM_HALITE_MAGNESITE_MIRABILITE_SYNGENITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
CALCITE_GLASERITE_HALITE_MAGNESITE_NAHCOLITE_THENARDITE<br />
HALITE_PIRSSONITE_THENARDITE_TRONA<br />
In each l<strong>in</strong>e, the list of m<strong>in</strong>erals corresponds to the whole sequence of precipit<strong>at</strong>ed m<strong>in</strong>erals dur<strong>in</strong>g concentr<strong>at</strong>ive evapor<strong>at</strong>ion.<br />
the way of <strong>in</strong>creas<strong>in</strong>g the solute load of the groundw<strong>at</strong>er. Precipit<strong>at</strong>ion<br />
and re-solution <strong>in</strong>evitably lead to strong segreg<strong>at</strong>ion of the <strong>in</strong>itial<br />
solutes. Therefore, sorption ion exchange and reduction reaction on<br />
m<strong>in</strong>eral surface may also remove certa<strong>in</strong> solute.<br />
8. Conclusions<br />
<strong>Lake</strong> <strong>Pikrolimni</strong> is a sal<strong>in</strong>e lake th<strong>at</strong> is characterized by alkal<strong>in</strong>e<br />
br<strong>in</strong>e, poor <strong>in</strong> Ca 2+ and Mg 2+ . The dilute HCO 3 − spr<strong>in</strong>g fresh w<strong>at</strong>er<br />
(350 g/L dissolved solid). Such br<strong>in</strong>es show a considerable<br />
range <strong>in</strong> ionic composition and concentr<strong>at</strong>ion. High solute concentr<strong>at</strong>ion<br />
due to solar evapor<strong>at</strong>ion of w<strong>at</strong>er, m<strong>in</strong>eral precipit<strong>at</strong>ion,<br />
fractional dissolution and solute recycl<strong>in</strong>g are the ma<strong>in</strong> processes<br />
responsible for these br<strong>in</strong>es form<strong>at</strong>ion. In particular, these evapor<strong>at</strong><strong>in</strong>g<br />
conditions were such th<strong>at</strong> <strong>in</strong>curred a hydrogeochemical environment<br />
th<strong>at</strong> was responsible for the lake to provide, <strong>in</strong> dry periods,<br />
“nitrum chalestricum” (trona). Besides, the progressive concentr<strong>at</strong>ion<br />
of br<strong>in</strong>es <strong>in</strong> alkal<strong>in</strong>e lakes leads to a preferential precipit<strong>at</strong>ion of<br />
sodium carbon<strong>at</strong>e followed by sulf<strong>at</strong>es and chlorides. This conclusion<br />
comes <strong>in</strong> agreement with the results. Also, the m<strong>in</strong>eralogical analysis<br />
(X-Ray Diffraction of salts), the evapor<strong>at</strong>ion simul<strong>at</strong>ion and the<br />
thermodynamic model showed th<strong>at</strong> salts of trona, burkeite and halite,<br />
deposit from these br<strong>in</strong>es. So, the conditions th<strong>at</strong> are responsible for<br />
the form<strong>at</strong>ion of soda seem to be present <strong>in</strong> the bas<strong>in</strong> and confirm<br />
Pl<strong>in</strong>y's description.<br />
Fig. 6. M<strong>in</strong>erals molarity versus concentr<strong>at</strong>ion factor.