A natron source at Pikrolimni Lake in Greece? Geochemical evidence
A natron source at Pikrolimni Lake in Greece? Geochemical evidence
A natron source at Pikrolimni Lake in Greece? Geochemical evidence
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
138 E. Dotsika et al. / Journal of <strong>Geochemical</strong> Explor<strong>at</strong>ion 103 (2009) 133–143<br />
to form from evapor<strong>at</strong><strong>in</strong>g w<strong>at</strong>er is calcite, and calcite precipit<strong>at</strong>ion<br />
cont<strong>in</strong>ues until either Ca 2+ or CO 3 2− is exhausted. W<strong>at</strong>ers with a<br />
low proportion of CO 3<br />
2−<br />
TOT rel<strong>at</strong>ive to Ca 2+ will be carbon<strong>at</strong>e depleted<br />
after calcite form<strong>at</strong>ion, and will thus not yield Na-carbon<strong>at</strong>e<br />
m<strong>in</strong>erals upon further evapor<strong>at</strong>ion. So the elev<strong>at</strong>ed alkal<strong>in</strong>ity/2[Ca 2+ ]<br />
r<strong>at</strong>io is a ma<strong>in</strong> requirement, accord<strong>in</strong>g to Hardie and Eugster (1970),for<br />
the form<strong>at</strong>ion of significant quantities of Na-carbon<strong>at</strong>e m<strong>in</strong>erals.<br />
Also, accord<strong>in</strong>g to Hardie and Eugster (1970) the appearance of sepiolite<br />
and gypsum determ<strong>in</strong>es the evapor<strong>at</strong>ive sequence. So, after the sepiolite<br />
precipit<strong>at</strong>ion the w<strong>at</strong>er can become carbon<strong>at</strong>e-enriched and alkal<strong>in</strong>e<br />
earth-poor or vice versa. If alkal<strong>in</strong>ity is higher than 2[Ca 2+ +Mg 2+ ]<br />
then the sepiolite precipit<strong>at</strong>ion is not capable to modify the evolution of<br />
the solution along the p<strong>at</strong>h of alkal<strong>in</strong>e facies (Risacher, 1992). The<br />
significantly high proportion of CO 3<br />
2−<br />
TOT rel<strong>at</strong>ive to 2[Ca 2+ +Mg 2+ ]<br />
<strong>in</strong> <strong>Pikrolimni</strong> area w<strong>at</strong>ers causes them to move along the p<strong>at</strong>h,<br />
<strong>in</strong> the Hardie–Eugster model, of alkal<strong>in</strong>e faces, form<strong>in</strong>g Na–CO 3<br />
m<strong>in</strong>erals.<br />
However, because the r<strong>at</strong>io of alkal<strong>in</strong>ity to Ca 2+ and Mg 2+ controls<br />
the types of evaporite m<strong>in</strong>erals formed, and specially the deposition of<br />
trona m<strong>in</strong>eral, it is very important to determ<strong>in</strong>e the potential<br />
processes th<strong>at</strong> affected the HCO 3 − contents <strong>in</strong> these w<strong>at</strong>ers.<br />
5.2. Potential processes affected the concentr<strong>at</strong>ion of HCO 3<br />
−<br />
There are a number of processes th<strong>at</strong> can affect the HCO − 3 contents <strong>in</strong><br />
groundw<strong>at</strong>er [these w<strong>at</strong>ers are rich <strong>in</strong> HCO − 3 (about 1.50–3 g/L)].<br />
Eugster (1980) described <strong>in</strong> detail processes th<strong>at</strong> can affect the<br />
concentr<strong>at</strong>ion of alkal<strong>in</strong>ity <strong>in</strong> groundw<strong>at</strong>er: dissolution or precipit<strong>at</strong>ion<br />
of carbon<strong>at</strong>e m<strong>in</strong>erals, chemical we<strong>at</strong>her<strong>in</strong>g of silic<strong>at</strong>es, redox reactions,<br />
especially the reduction of NO − 3 to NH + 4 and SO 2− 4 to HS − , microbial<br />
respir<strong>at</strong>ion or anaerobic decay and the conversion of CO 2 of deep orig<strong>in</strong><br />
to HCO − 3 <strong>in</strong> the aquifer. The dissolution/precipit<strong>at</strong>ion of carbon<strong>at</strong>e<br />
m<strong>in</strong>eral is a common control of HCO − 3 contents <strong>in</strong> groundw<strong>at</strong>er. These<br />
w<strong>at</strong>ers show an important over-s<strong>at</strong>ur<strong>at</strong>ion with respect to calcite<br />
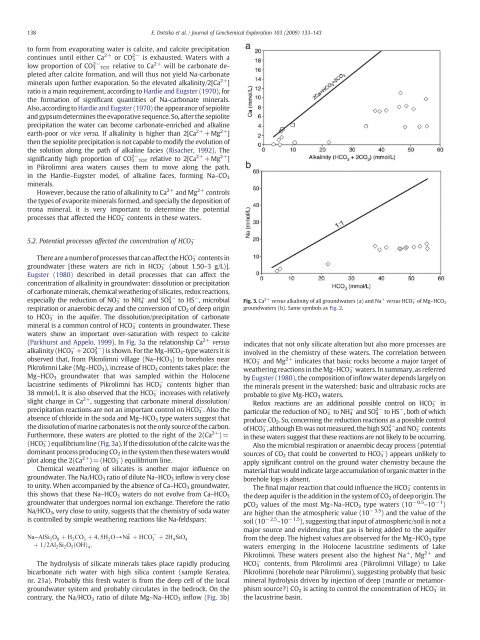
(Parkhurst and Appelo, 1999). In Fig. 3a the rel<strong>at</strong>ionship Ca 2+ versus<br />
alkal<strong>in</strong>ity (HCO − 3 +2CO 2− 3 ) is shown. For the Mg–HCO 3 -type w<strong>at</strong>ers it is<br />
observed th<strong>at</strong>, from <strong>Pikrolimni</strong> village (Na–HCO 3 ) to boreholes near<br />
<strong>Pikrolimni</strong> <strong>Lake</strong> (Mg–HCO 3 ), <strong>in</strong>crease of HCO 3 contents takes place: the<br />
Mg–HCO 3 groundw<strong>at</strong>er th<strong>at</strong> was sampled with<strong>in</strong> the Holocene<br />
lacustr<strong>in</strong>e sediments of <strong>Pikrolimni</strong> has HCO − 3 contents higher than<br />
38 mmol/L. It is also observed th<strong>at</strong> the HCO − 3 <strong>in</strong>creases with rel<strong>at</strong>ively<br />
slight change <strong>in</strong> Ca 2+ , suggest<strong>in</strong>g th<strong>at</strong> carbon<strong>at</strong>e m<strong>in</strong>eral dissolution/<br />
precipit<strong>at</strong>ion reactions are not an important control on HCO − 3 .Alsothe<br />
absence of chloride <strong>in</strong> the soda and Mg–HCO 3 type w<strong>at</strong>ers suggest th<strong>at</strong><br />
the dissolution of mar<strong>in</strong>e carbon<strong>at</strong>es is not the only <strong>source</strong> of the carbon.<br />
Furthermore, these w<strong>at</strong>ers are plotted to the right of the 2(Ca 2+ )=<br />
(HCO − 3 ) equilibrium l<strong>in</strong>e (Fig. 3a). If the dissolution of the calcite was the<br />
dom<strong>in</strong>ant process produc<strong>in</strong>g CO 2 <strong>in</strong> the system then these w<strong>at</strong>ers would<br />
plot along the 2(Ca 2+ )=(HCO − 3 ) equilibrium l<strong>in</strong>e.<br />
Chemical we<strong>at</strong>her<strong>in</strong>g of silic<strong>at</strong>es is another major <strong>in</strong>fluence on<br />
groundw<strong>at</strong>er. The Na/HCO 3 r<strong>at</strong>io of dilute Na–HCO 3 <strong>in</strong>flow is very close<br />
to unity. When accompanied by the absence of Ca–HCO 3 groundw<strong>at</strong>er,<br />
this shows th<strong>at</strong> these Na–HCO 3 w<strong>at</strong>ers do not evolve from Ca–HCO 3<br />
groundw<strong>at</strong>er th<strong>at</strong> undergoes normal ion exchange. Therefore the r<strong>at</strong>io<br />
Na/HCO 3 , very close to unity, suggests th<strong>at</strong> the chemistry of soda w<strong>at</strong>er<br />
is controlled by simple we<strong>at</strong>her<strong>in</strong>g reactions like Na-feldspars:<br />
Na–AlSi 3 O 8 þ H 2 CO 3 þ 4; 5H 2 O→Na þ þ HCO − 3 þ 2H 4 SiO 4<br />
þ 1=2Al 2 Si 2 O 5 ðOHÞ 4<br />
:<br />
The hydrolysis of silic<strong>at</strong>e m<strong>in</strong>erals takes place rapidly produc<strong>in</strong>g<br />
bicarbon<strong>at</strong>e rich w<strong>at</strong>er with high silica content (sample Ker<strong>at</strong>ea,<br />
nr. 21a). Probably this fresh w<strong>at</strong>er is from the deep cell of the local<br />
groundw<strong>at</strong>er system and probably circul<strong>at</strong>es <strong>in</strong> the bedrock. On the<br />
contrary, the Na/HCO 3 r<strong>at</strong>io of dilute Mg–Na–HCO 3 <strong>in</strong>flow (Fig. 3b)<br />
Fig. 3. Ca 2+ versus alkal<strong>in</strong>ity of all groundw<strong>at</strong>ers (a) and Na + versus HCO 3 − of Mg–HCO 3<br />
groundw<strong>at</strong>ers (b). Same symbols as Fig. 2.<br />
<strong>in</strong>dic<strong>at</strong>es th<strong>at</strong> not only silic<strong>at</strong>e alter<strong>at</strong>ion but also more processes are<br />
<strong>in</strong>volved <strong>in</strong> the chemistry of these w<strong>at</strong>ers. The correl<strong>at</strong>ion between<br />
HCO 3 − and Mg 2+ <strong>in</strong>dic<strong>at</strong>es th<strong>at</strong> basic rocks become a major target of<br />
we<strong>at</strong>her<strong>in</strong>g reactions <strong>in</strong> the Mg–HCO 3 − w<strong>at</strong>ers. In summary, as referred<br />
by Eugster (1980), the composition of <strong>in</strong>flow w<strong>at</strong>er depends largely on<br />
the m<strong>in</strong>erals present <strong>in</strong> the w<strong>at</strong>ershed: basic and ultrabasic rocks are<br />
probable to give Mg-HCO 3 w<strong>at</strong>ers.<br />
Redox reactions are an additional possible control on HCO 3 − <strong>in</strong><br />
particular the reduction of NO 3 − to NH 4 + and SO 4 2− to HS − ,bothofwhich<br />
produce CO 2 . So, concern<strong>in</strong>g the reduction reactions as a possible control<br />
of HCO 3 − , although Eh was not measured, the high SO 4 2− and NO 3 − contents<br />
<strong>in</strong> these w<strong>at</strong>ers suggest th<strong>at</strong> these reactions are not likely to be occurr<strong>in</strong>g.<br />
Also the microbial respir<strong>at</strong>ion or anaerobic decay process (potential<br />
<strong>source</strong>s of CO 2 th<strong>at</strong> could be converted to HCO 3 − ) appears unlikely to<br />
apply significant control on the ground w<strong>at</strong>er chemistry because the<br />
m<strong>at</strong>erial th<strong>at</strong> would <strong>in</strong>dic<strong>at</strong>e large accumul<strong>at</strong>ion of organic m<strong>at</strong>ter <strong>in</strong> the<br />
borehole logs is absent.<br />
The f<strong>in</strong>al major reaction th<strong>at</strong> could <strong>in</strong>fluence the HCO 3 − contents <strong>in</strong><br />
the deep aquifer is the addition <strong>in</strong> the system of CO 2 of deep orig<strong>in</strong>. The<br />
pCO 2 values of the most Mg–Na–HCO 3 type w<strong>at</strong>ers (10 − 0.5 –10 − 1 )<br />
are higher than the <strong>at</strong>mospheric value (10 − 3.5 ) and the value of the<br />
soil (10 − 2.5 –10 − 1.5 ), suggest<strong>in</strong>g th<strong>at</strong> <strong>in</strong>put of <strong>at</strong>mospheric/soil is not a<br />
major <strong>source</strong> and evidenc<strong>in</strong>g th<strong>at</strong> gas is be<strong>in</strong>g added to the aquifer<br />
from the deep. The highest values are observed for the Mg–HCO 3 type<br />
w<strong>at</strong>ers emerg<strong>in</strong>g <strong>in</strong> the Holocene lacustr<strong>in</strong>e sediments of <strong>Lake</strong><br />
<strong>Pikrolimni</strong>. These w<strong>at</strong>ers present also the highest Na + ,Mg 2+ and<br />
HCO 3 − contents, from <strong>Pikrolimni</strong> area (<strong>Pikrolimni</strong> Village) to <strong>Lake</strong><br />
<strong>Pikrolimni</strong> (borehole near <strong>Pikrolimni</strong>), suggest<strong>in</strong>g probably th<strong>at</strong> basic<br />
m<strong>in</strong>eral hydrolysis driven by <strong>in</strong>jection of deep (mantle or metamorphism<br />
<strong>source</strong>) CO 2 is act<strong>in</strong>g to control the concentr<strong>at</strong>ion of HCO 3 − <strong>in</strong><br />
the lacustr<strong>in</strong>e bas<strong>in</strong>.