Introductory Guidance on the CLP Regulation - ECHA - Europa
Introductory Guidance on the CLP Regulation - ECHA - Europa
Introductory Guidance on the CLP Regulation - ECHA - Europa
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Basic guidance to Regulati<strong>on</strong> (EC) No 1272/2008 <strong>on</strong> Classificati<strong>on</strong>, Labelling and Packaging of substances and mixtures<br />
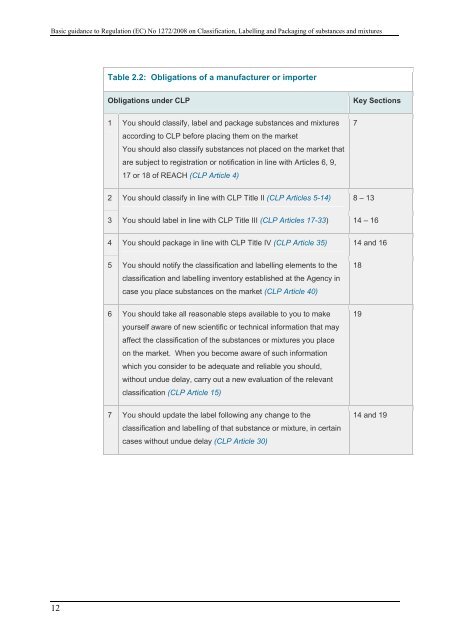
Table 2.2: Obligati<strong>on</strong>s of a manufacturer or importer<br />
Obligati<strong>on</strong>s under <strong>CLP</strong><br />
Key Secti<strong>on</strong>s<br />
1 You should classify, label and package substances and mixtures<br />
according to <strong>CLP</strong> before placing <strong>the</strong>m <strong>on</strong> <strong>the</strong> market<br />
You should also classify substances not placed <strong>on</strong> <strong>the</strong> market that<br />
are subject to registrati<strong>on</strong> or notificati<strong>on</strong> in line with Articles 6, 9,<br />
17 or 18 of REACH (<strong>CLP</strong> Article 4)<br />
7<br />
2 You should classify in line with <strong>CLP</strong> Title II (<strong>CLP</strong> Articles 5-14) 8 – 13<br />
3 You should label in line with <strong>CLP</strong> Title III (<strong>CLP</strong> Articles 17-33) 14 – 16<br />
4 You should package in line with <strong>CLP</strong> Title IV (<strong>CLP</strong> Article 35) 14 and 16<br />
5 You should notify <strong>the</strong> classificati<strong>on</strong> and labelling elements to <strong>the</strong><br />
classificati<strong>on</strong> and labelling inventory established at <strong>the</strong> Agency in<br />
case you place substances <strong>on</strong> <strong>the</strong> market (<strong>CLP</strong> Article 40)<br />
18<br />
6 You should take all reas<strong>on</strong>able steps available to you to make<br />
yourself aware of new scientific or technical informati<strong>on</strong> that may<br />
affect <strong>the</strong> classificati<strong>on</strong> of <strong>the</strong> substances or mixtures you place<br />
<strong>on</strong> <strong>the</strong> market. When you become aware of such informati<strong>on</strong><br />
which you c<strong>on</strong>sider to be adequate and reliable you should,<br />
without undue delay, carry out a new evaluati<strong>on</strong> of <strong>the</strong> relevant<br />
classificati<strong>on</strong> (<strong>CLP</strong> Article 15)<br />
19<br />
7 You should update <strong>the</strong> label following any change to <strong>the</strong><br />
classificati<strong>on</strong> and labelling of that substance or mixture, in certain<br />
cases without undue delay (<strong>CLP</strong> Article 30)<br />
14 and 19<br />
12