Introductory Guidance on the CLP Regulation - ECHA - Europa
Introductory Guidance on the CLP Regulation - ECHA - Europa
Introductory Guidance on the CLP Regulation - ECHA - Europa
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Basic guidance to Regulati<strong>on</strong> (EC) No 1272/2008 <strong>on</strong> Classificati<strong>on</strong>, Labelling and Packaging of substances and mixtures<br />
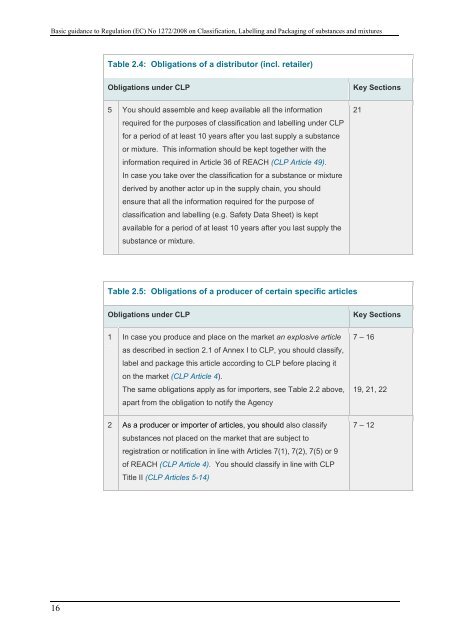
Table 2.4: Obligati<strong>on</strong>s of a distributor (incl. retailer)<br />
Obligati<strong>on</strong>s under <strong>CLP</strong><br />
Key Secti<strong>on</strong>s<br />
5 You should assemble and keep available all <strong>the</strong> informati<strong>on</strong><br />
required for <strong>the</strong> purposes of classificati<strong>on</strong> and labelling under <strong>CLP</strong><br />
for a period of at least 10 years after you last supply a substance<br />
or mixture. This informati<strong>on</strong> should be kept toge<strong>the</strong>r with <strong>the</strong><br />
informati<strong>on</strong> required in Article 36 of REACH (<strong>CLP</strong> Article 49).<br />
In case you take over <strong>the</strong> classificati<strong>on</strong> for a substance or mixture<br />
derived by ano<strong>the</strong>r actor up in <strong>the</strong> supply chain, you should<br />
ensure that all <strong>the</strong> informati<strong>on</strong> required for <strong>the</strong> purpose of<br />
classificati<strong>on</strong> and labelling (e.g. Safety Data Sheet) is kept<br />
available for a period of at least 10 years after you last supply <strong>the</strong><br />
substance or mixture.<br />
21<br />
Table 2.5: Obligati<strong>on</strong>s of a producer of certain specific articles<br />
Obligati<strong>on</strong>s under <strong>CLP</strong><br />
Key Secti<strong>on</strong>s<br />
1 In case you produce and place <strong>on</strong> <strong>the</strong> market an explosive article<br />
as described in secti<strong>on</strong> 2.1 of Annex I to <strong>CLP</strong>, you should classify,<br />
label and package this article according to <strong>CLP</strong> before placing it<br />
<strong>on</strong> <strong>the</strong> market (<strong>CLP</strong> Article 4).<br />
The same obligati<strong>on</strong>s apply as for importers, see Table 2.2 above,<br />
apart from <strong>the</strong> obligati<strong>on</strong> to notify <strong>the</strong> Agency<br />
7 – 16<br />
19, 21, 22<br />
2 As a producer or importer of articles, you should also classify<br />
substances not placed <strong>on</strong> <strong>the</strong> market that are subject to<br />
registrati<strong>on</strong> or notificati<strong>on</strong> in line with Articles 7(1), 7(2), 7(5) or 9<br />
of REACH (<strong>CLP</strong> Article 4). You should classify in line with <strong>CLP</strong><br />
Title II (<strong>CLP</strong> Articles 5-14)<br />
7 – 12<br />
16