Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
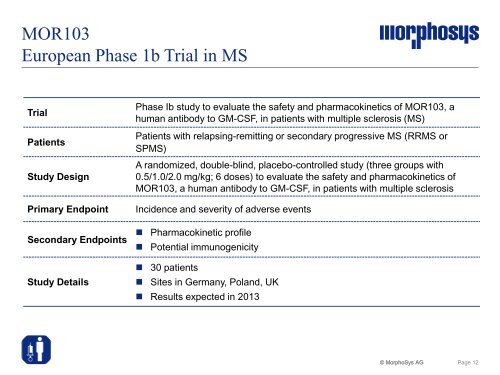
MOR103<br />
European Phase 1b Trial in MS<br />
Trial<br />
Patients<br />
Study Design<br />
Phase Ib study to evaluate the safety and pharmacokinetics of MOR103, a<br />
human antibody to GM-CSF, in patients with multiple sclerosis (MS)<br />
Patients with relapsing-remitting or secondary progressive MS (RRMS or<br />
SPMS)<br />
A randomized, double-blind, placebo-controlled study (three groups with<br />
0.5/1.0/2.0 mg/kg; 6 doses) to evaluate the safety and pharmacokinetics of<br />
MOR103, a human antibody to GM-CSF, in patients with multiple sclerosis<br />
Primary Endpoint Incidence and severity of adverse events<br />
Secondary Endpoints<br />
Study Details<br />
� Pharmacokinetic profile<br />
� Potential immunogenicity<br />
� 30 patients<br />
� Sites in Germany, Poland, UK<br />
� Results expected in 2013<br />
© <strong>MorphoSys</strong> AG<br />
Page 12