Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
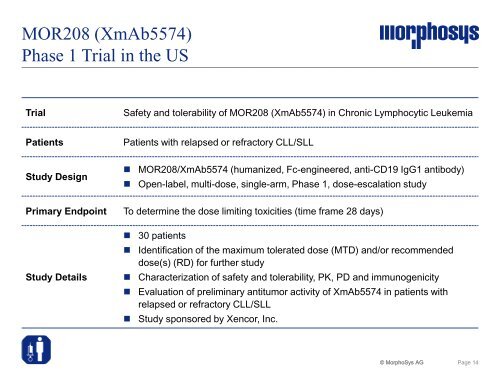
MOR208 (XmAb5574)<br />
Phase 1 Trial in the US<br />
Trial Safety and tolerability of MOR208 (XmAb5574) in Chronic Lymphocytic Leukemia<br />
Patients Patients with relapsed or refractory CLL/SLL<br />
Study Design<br />
� MOR208/XmAb5574 (humanized, Fc-engineered, anti-CD19 IgG1 antibody)<br />
� Open-label, multi-dose, single-arm, Phase 1, dose-escalation study<br />
Primary Endpoint To determine the dose limiting toxicities (time frame 28 days)<br />
Study Details<br />
� 30 patients<br />
� Identification of the maximum tolerated dose (MTD) and/or recommended<br />
dose(s) (RD) for further study<br />
� Characterization of safety and tolerability, PK, PD and immunogenicity<br />
� Evaluation of preliminary antitumor activity of XmAb5574 in patients with<br />
relapsed or refractory CLL/SLL<br />
� Study sponsored by Xencor, Inc.<br />
© <strong>MorphoSys</strong> AG<br />
Page 14