Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
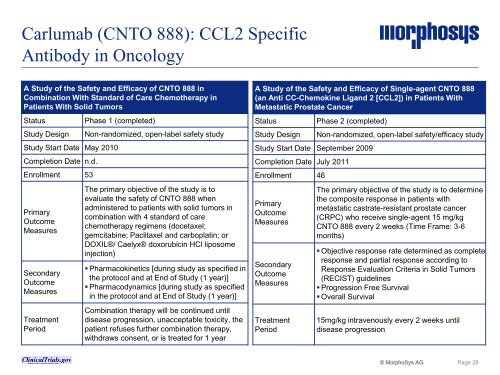
Carlumab (CNTO 888): CCL2 Specific<br />
Antibody in Oncology<br />
A Study of the Safety and Efficacy of CNTO 888 in<br />
Combination With Standard of Care Chemotherapy in<br />
Patients With Solid Tumors<br />
Status Phase 1 (completed)<br />
Study Design Non-randomized, open-label safety study<br />
Study Start Date May 2010<br />
Completion Date n.d.<br />
Enrollment 53<br />
Primary<br />
Outcome<br />
Measures<br />
Secondary<br />
Outcome<br />
Measures<br />
Treatment<br />
Period<br />
The primary objective of the study is to<br />
evaluate the safety of CNTO 888 when<br />
administered to patients with solid tumors in<br />
combination with 4 standard of care<br />
chemotherapy regimens (docetaxel;<br />
gemcitabine; Paclitaxel and carboplatin; or<br />
DOXIL®/ Caelyx® doxorubicin HCl liposome<br />
injection)<br />
� Pharmacokinetics [during study as specified in<br />
the protocol and at End of Study (1 year)]<br />
� Pharmacodynamics [during study as specified<br />
in the protocol and at End of Study (1 year)]<br />
Combination therapy will be continued until<br />
disease progression, unacceptable toxicity, the<br />
patient refuses further combination therapy,<br />
withdraws consent, or is treated for 1 year<br />
A Study of the Safety and Efficacy of Single-agent CNTO 888<br />
(an Anti CC-Chemokine Ligand 2 [CCL2]) in Patients With<br />
Metastatic Prostate Cancer<br />
Status Phase 2 (completed)<br />
Study Design Non-randomized, open-label safety/efficacy study<br />
Study Start Date September 2009<br />
Completion Date July 2011<br />
Enrollment 46<br />
Primary<br />
Outcome<br />
Measures<br />
Secondary<br />
Outcome<br />
Measures<br />
Treatment<br />
Period<br />
The primary objective of the study is to determine<br />
the composite response in patients with<br />
metastatic castrate-resistant prostate cancer<br />
(CRPC) who receive single-agent 15 mg/kg<br />
CNTO 888 every 2 weeks (Time Frame: 3-6<br />
months)<br />
� Objective response rate determined as complete<br />
response and partial response according to<br />
Response Evaluation Criteria in Solid Tumors<br />
(RECIST) guidelines<br />
� Progression Free Survival<br />
� Overall Survival<br />
15mg/kg intravenously every 2 weeks until<br />
disease progression<br />
© <strong>MorphoSys</strong> AG<br />
Page 28