You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
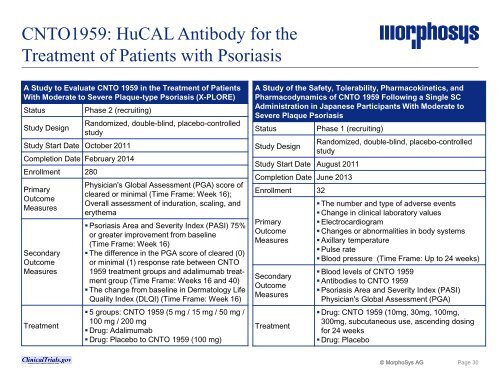
CNTO1959: HuCAL Antibody for the<br />
Treatment of Patients with Psoriasis<br />
A Study to Evaluate CNTO 1959 in the Treatment of Patients<br />
With Moderate to Severe Plaque-type Psoriasis (X-PLORE)<br />
Status Phase 2 (recruiting)<br />
Study Design<br />
Study Start Date October 2011<br />
Completion Date February 2014<br />
Enrollment 280<br />
Primary<br />
Outcome<br />
Measures<br />
Secondary<br />
Outcome<br />
Measures<br />
Treatment<br />
Randomized, double-blind, placebo-controlled<br />
study<br />
Physician's Global Assessment (PGA) score of<br />
cleared or minimal (Time Frame: Week 16);<br />
Overall assessment of induration, scaling, and<br />
erythema<br />
� Psoriasis Area and Severity Index (PASI) 75%<br />
or greater improvement from baseline<br />
(Time Frame: Week 16)<br />
� The difference in the PGA score of cleared (0)<br />
or minimal (1) response rate between CNTO<br />
1959 treatment groups and adalimumab treatment<br />
group (Time Frame: Weeks 16 and 40)<br />
� The change from baseline in Dermatology Life<br />
Quality Index (DLQI) (Time Frame: Week 16)<br />
� 5 groups: CNTO 1959 (5 mg / 15 mg / 50 mg /<br />
100 mg / 200 mg<br />
� Drug: Adalimumab<br />
� Drug: Placebo to CNTO 1959 (100 mg)<br />
A Study of the Safety, Tolerability, Pharmacokinetics, and<br />
Pharmacodynamics of CNTO 1959 Following a Single SC<br />
Administration in Japanese Participants With Moderate to<br />
Severe Plaque Psoriasis<br />
Status Phase 1 (recruiting)<br />
Study Design<br />
Study Start Date August 2011<br />
Completion Date June 2013<br />
Enrollment 32<br />
Primary<br />
Outcome<br />
Measures<br />
Secondary<br />
Outcome<br />
Measures<br />
Treatment<br />
Randomized, double-blind, placebo-controlled<br />
study<br />
� The number and type of adverse events<br />
� Change in clinical laboratory values<br />
� Electrocardiogram<br />
� Changes or abnormalities in body systems<br />
� Axillary temperature<br />
� Pulse rate<br />
� Blood pressure (Time Frame: Up to 24 weeks)<br />
� Blood levels of CNTO 1959<br />
� Antibodies to CNTO 1959<br />
� Psoriasis Area and Severity Index (PASI)<br />
Physician's Global Assessment (PGA)<br />
� Drug: CNTO 1959 (10mg, 30mg, 100mg,<br />
300mg, subcutaneous use, ascending dosing<br />
for 24 weeks<br />
� Drug: Placebo<br />
© <strong>MorphoSys</strong> AG<br />
Page 30