The Students' Guide in Quantitative Analysis - Free Ebooks Library
The Students' Guide in Quantitative Analysis - Free Ebooks Library
The Students' Guide in Quantitative Analysis - Free Ebooks Library
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
34<br />
QUANTITATIVE ANALYSTS.<br />
N.B.—Make a strong hot solution of CuS0,,-t-.5H,0, add<br />
pieces of pumice-stone, boil hard, evaporate to dryness and<br />
ignite well. <strong>The</strong> product should be nearly white.<br />
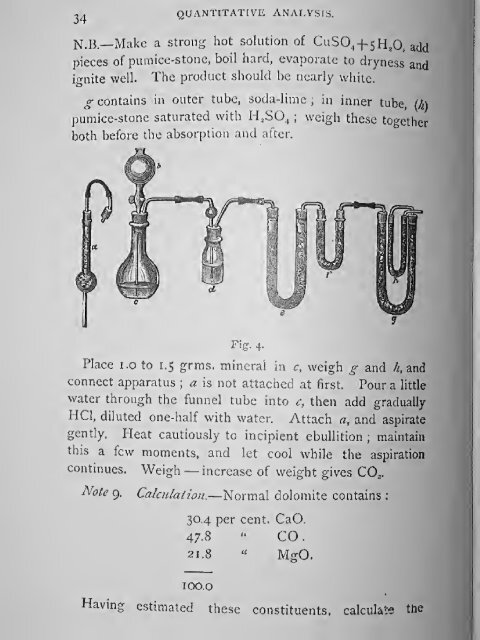
g conta<strong>in</strong>s <strong>in</strong> outer tube, soda-lime ; <strong>in</strong> <strong>in</strong>ner tube, {k\<br />
pumice-stone saturated with II^SO^ ;<br />
both before the absorption and after.<br />
Fig. 4.<br />
weigh these together<br />
Place i.o to 1.5 grms. m<strong>in</strong>eral <strong>in</strong> c, weigh g and h, and<br />
connect apparatus ; a is not attached at first. Pour a little<br />
water through the funnel tube <strong>in</strong>to c, then add gradually<br />
HCl, diluted one-half with water. Attach a, and aspirate<br />
gently. Heat cautiously to <strong>in</strong>cipient ebullition ; ma<strong>in</strong>ta<strong>in</strong><br />
this a few moments, and let cool while the aspiration<br />
cont<strong>in</strong>ues. Weigh — <strong>in</strong>crease of weight gives CO^.<br />
Note 9, Calculation.—Normal dolomite conta<strong>in</strong>s ;<br />
30.4 per cent. CaO.<br />
47.8 " CO.<br />
21.8 MgO.<br />
100.0<br />
Hav<strong>in</strong>g estimated these constituents, calculate the