Atmospheric Transmission Beer's Law
Atmospheric Transmission Beer's Law
Atmospheric Transmission Beer's Law
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
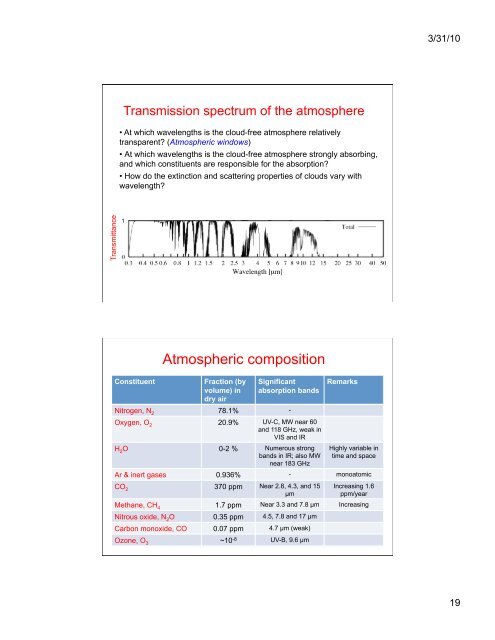
3/31/10<strong>Transmission</strong> spectrum of the atmosphere• At which wavelengths is the cloud-free atmosphere relativelytransparent? (<strong>Atmospheric</strong> windows)• At which wavelengths is the cloud-free atmosphere strongly absorbing,and which constituents are responsible for the absorption?• How do the extinction and scattering properties of clouds vary withwavelength?Transmittance1Constituent<strong>Atmospheric</strong> compositionFraction (byvolume) indry airSignificantabsorption bandsNitrogen, N 2 78.1% -Oxygen, O 2 20.9% UV-C, MW near 60and 118 GHz, weak inVIS and IRH 2 O 0-2 % Numerous strongbands in IR; also MWnear 183 GHzRemarksHighly variable intime and spaceAr & inert gases 0.936% - monoatomicCO 2 370 ppm Near 2.8, 4.3, and 15µmIncreasing 1.6ppm/yearMethane, CH 4 1.7 ppm Near 3.3 and 7.8 µm IncreasingNitrous oxide, N 2 O 0.35 ppm 4.5, 7.8 and 17 µmCarbon monoxide, CO 0.07 ppm 4.7 µm (weak)Ozone, O 3 ~10 -8 UV-B, 9.6 µm19