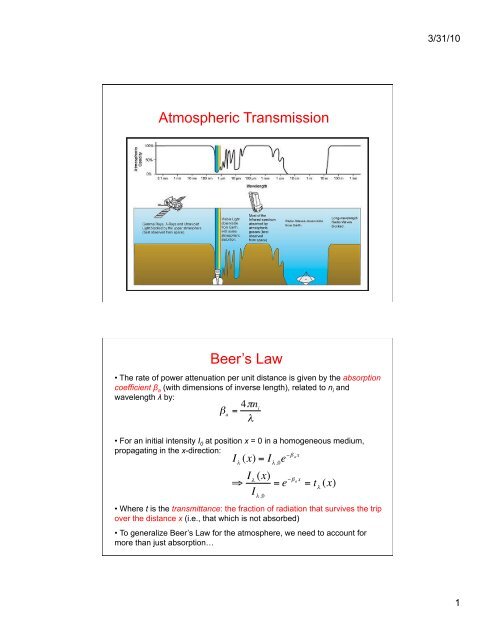
Atmospheric Transmission Beer's Law
Atmospheric Transmission Beer's Law
Atmospheric Transmission Beer's Law
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3/31/10<strong>Atmospheric</strong> <strong>Transmission</strong>Beer’s <strong>Law</strong>• The rate of power attenuation per unit distance is given by the absorptioncoefficient β a (with dimensions of inverse length), related to n i andwavelength λ by:• For an initial intensity I 0 at position x = 0 in a homogeneous medium,propagating in the x-direction:€β a= 4πn iλI λ(x) = I λ ,0e −β a x⇒ I λ (x)I λ ,0= e −β a x= t λ(x)• Where t is the transmittance: the fraction of radiation that survives the tripover the distance x (i.e., that which is not absorbed)• To generalize Beer’s <strong>Law</strong> for the atmosphere, we need to account formore than just absorption… €1
3/31/10Extinction of radiationMilk, ink and water in dishes on an overhead projectorTop view (reflected light)Projected view (transmitted light)• Dark shadows for milk (scattering medium) and ink(absorbing medium) show that absorption and scatteringare equally effective at depleting transmitted radiationBlack clouds are not absorbing…• The appearance of clouds depends onwhether they are viewed by reflected(scattered) or transmitted light• In the latter case, clouds appear darkagainst a bright background due toattenuation (scattering) of light by thecloud (though only for thick clouds)2
3/31/10Extinction of radiation• A beam of radiation can be attenuated (extinguished)not only by absorption but also by scattering• The reduction of intensity of a beam of radiation due toabsorption and scattering is called extinction• Hence we define a more general extinction coefficient(β e ) to replace the absorption coefficient (β a )encountered earlier• β e = β a + β s…we also introduce a scattering coefficient (β s )What is scattering?When light encounters matter, matternot only re-emits light in the forwarddirection, but it also re-emits light inall other directions.Light sourceMoleculeThis is called scattering – theredirection of radiation out of theoriginal direction of propagation.Contrast with absorption, whichinvolves conversion of EM energy toheat or chemical energy.Light scattering is everywhere. All molecules scatter light. Surfacesscatter light. Scattering causes milk and clouds to be white and thedaytime sky to be blue. It is the basis of nearly all optical phenomena.3
3/31/10Scattering geometryNote variation ofintensity withdirectionBackward scattering(backscattering)Forward scatteringThin clouds – effect of scatteringThin cloudsThick clouds• Optically thin clouds can look brighter when viewed towards the Sun, andvice versa• This is due to the extreme asymmetry in forward-backward scattering bycloud droplets, and the dominance of single (not multiple) scattering4
3/31/10• β e = β a + β sExtinction of radiation• The extinction coefficient (β e ) is the sum of an absorptioncoefficient (β a ) and a scattering coefficient (β s ) – all have unitsof inverse length (m -1 )• For milk, β a ≈ 0 so β e ≈ β s ; for ink β s ≈ 0 so β e ≈ β a• To characterize the relative importance of scattering andabsorption in a medium, the single scatter albedo (ω) is used:• ω = 0 for a purely absorbing medium (ink), 1 in a purelyscattering medium (milk)Based on their appearance what would you concludeabout the spectral dependence of β e and ω for……• A cloud?Highly reflective ofsunlight, suggesting thatthe SSA is very close toone. Clouds are white,rather than some othershade, suggesting thatthe SSA and extinctioncoefficient don’t dependmuch on wavelengthwithin the visible band.5
3/31/10Based on their appearance what would you concludeabout the spectral dependence of β e and ω for……• Diesel exhaust fumes?Scatters some light, butappear rather dark gray,suggesting that the SSAis greater than zero butmuch less than one.Color is rather neutral, sospectral dependence issmall.Based on their appearance what would you concludeabout the spectral dependence of β e and ω for……• A cloud-free atmosphere?The setting sun (which is seenvia a rather long path throughthe atmosphere) is reddish,suggesting that extinction isgreater for shorter wavelengths.Much of the radiation that isextinguished is seen in otherdirections as scattered radiation-- i.e., the blue sky; hence, weconclude that the SSA isreasonably close to unity.6
3/31/10Properties of Beer’s <strong>Law</strong>• For propagation of radiation over an optical path τ
3/31/10Optical depthThe optical depth expresses the quantity of light removed from a beam by absorptionor scattering during its path through a medium.If τ = 0.5, transmissivity = ~60%, if τ = 1, t = ~37% and if τ = 2.5, t = ~8%.If τ > 1, the medium is optically thickOptical depthIf a gas is optically thick, a photon will interact many,many times with particles before it finally escapesfrom the cloud. Any photon entering the cloud willhave its direction changed many times by scattering –so its ‘output’ direction has nothing to do with its‘input’ direction. We can't see anything at all throughthe cloud: it is opaque.You can't see through an optically thick medium; youcan only see light emitted by the very outermostlayers. But there is one convenient feature of opticallythick materials: the spectrum of the light they emit is ablackbody spectrum, or very close to it.9
3/31/10Optical depth problemThe optical depth of the stratospheric aerosol layer is typically about 1 x 10 -4 forwavelengths of 1 µm. Following the eruption of Mt. Pinatubo volcano in June1991, the optical depth at 1 µm increased to 1 x 10 -2 . How much more or lessinfrared radiation was transmitted through this layer? What do you think theconsequences of this change might be?Pinatubo eruption, June 1991 Space Shuttle view of aerosol veil, August 1991From the restatement of <strong>Beer's</strong> <strong>Law</strong>, we know that I/I 0 = exp (-τ), where the ratioI/I 0 represents the fraction of light transmitted through the medium.We can make use of an approximation: for small values of x, e -x ≈ (1 - x). Bothvalues for the optical depth are quite small, so we can approximate:Typical: I/I 0 = e -(0.0001) ≈ (1 - 0.0001) ≈ 1Optical depth problemPost-volcano: I/I 0 = e -(0.01) ≈ (1 - 0.01) ≈ 0.99The amount of light transmitted through the aerosol layer decreased followingthe volcanic eruption because the optical depth increased. Approximately 1%less light was transmitted. That missing 1% had to go somewhere - either bescattered by the particles or absorbed by them. In this case, it was likelyabsorbed.Evidence has shown that the enhanced aerosol layer following the Pinatuboeruption was responsible for slightly increasing the temperature of the lowerstratosphere..10
3/31/10More Beer’s <strong>Law</strong>Also known as the Beer-Lambert <strong>Law</strong> or the Beer-Bouguer-Lambert <strong>Law</strong>, orother combinations…I 0βdIt = II 0= e −βdThe law states that there is a logarithmic dependence between the transmission (ortransmissivity), t, of light through a substance and the product of the absorptioncoefficient (β) and the distance the light travels through the material, or the pathlength (d).€More Beer’s <strong>Law</strong>For a gaseous absorber, the absorption coefficient (β) is written as theproduct of an absorption cross-section (σ, cm 2 ) and the number density ofabsorbers (molecules cm -3 ):I 0σ, NdIt = II 0= e −σNd(For liquids the absorption coefficient is the product of the molar absorptivity of theabsorber and the concentration of the absorber)€11
3/31/10More Beer’s <strong>Law</strong>Now taking the natural logarithm (the convention for atmospheric science):⎛⎛⇒ ln I ⎞⎞⎜⎜ ⎟⎟ = −σNd⎝⎝ ⎠⎠I 0⎛⎛⇒ A = −ln I ⎞⎞ ⎛⎛⎜⎜ ⎟⎟ = ln I ⎞⎞0⎜⎜ ⎟⎟ = σNd⎝⎝ ⎠⎠ ⎝⎝ I ⎠⎠I 0Deviation at high concentrations€Where A is the absorbance (negative logarithm of transmittance).100% transmittance = absorbance of zero; 10% transmittance = absorbance of 2.3Hence Beer’s <strong>Law</strong> states that there is a linear relationship between absorbance andconcentration (as opposed to a logarithmic dependence on transmittance).If the path length (d) and absorption cross-section are known and the absorbance ismeasured, the number density of absorbers can be deduced.Derivation of Beer’s <strong>Law</strong>The Beer-Lambert law can be derived by approximating the absorbing molecule byan opaque disk whose cross-sectional area, σ, represents the effective area seen bya photon of wavelength λ. Taking an infinitesimal slab, dz, of sample:I o is the intensity entering the sample at z = 0, I z is the intensity entering theinfinitesimal slab at z, dI is the intensity absorbed in the slab, and I is the intensity oflight leaving the sample.Then, the total opaque area on the slab due to the absorbers is: σ * N * A * dz.The fraction of photons absorbed is therefore: σ * N * A * dz / A = σ N dz12
3/31/10The fraction of photons absorbed by the slab is:(negative since photons are removed)Integrating both sides gives:Derivation of Beer’s <strong>Law</strong>dI= −σN dzI zlnI z= −σNz + CThe difference in intensity between z = 0 and z = b is therefore:Or for liquids:lnI€0− ln I = (−σN0 + C) − (−σNb + C) = σNb€ ⎛⎛⇒ ln I ⎞⎞0⎜⎜ ⎟⎟ = A = σNb⎝⎝ I ⎠⎠⎛⎛ IA = log 0⎞⎞10 ⎜⎜ ⎟⎟ = σNb⎝⎝ I ⎠⎠ 2.303 = εcb€Where ε = molar absorptivity (L mol -1 cm -1 or mol -1 cm 2 ) and c = concentration (mol cm -3 )€Deviations from Beer’s <strong>Law</strong>At least five conditions need to be satisfied in order for Beer’s law to bevalid. These are:• The absorbers must act independently of each other.• The absorbing medium must be homogeneously distributed in theinteraction volume and must not scatter the radiation.• The incident radiation must consist of parallel rays, each traversing thesame length in the absorbing medium.• The incident radiation should preferably be monochromatic, or have atleast a width that is narrower than the absorbing transition.• The incident flux must not influence the atoms or molecules; it should onlyact as a non-invasive probe of the species under study.• If any of these conditions is not fulfilled, there will be deviations fromBeer’s law.13
3/31/10Deviations from Beer’s <strong>Law</strong>reflectionI 0absorptionreflectiondirectdiffuse(scattered)Direct Beam:I(x) = I 0 exp(- β ext x)<strong>Transmission</strong> Coefficient for the DirectBeam:t direct = exp(- β ext x)xBeer’s <strong>Law</strong> applies to the directbeam onlyMass extinction coefficient• The extinction coefficient β e expresses extinction by reference to ageometric distance (or path length) through a medium• Problem: The measured transmission though a ink-water solution is 70%and the depth of the solution is 10 cm. Calculate β e .• What happens if the depth of the solution is doubled without addition of ink?• In remote sensing, we are usually interested in the mass of absorbingmaterial present, or the number of absorbing particles.• We define the mass extinction coefficient k e , relating the density of theabsorbing material (ρ) to β e :β e= ρk e€14
3/31/10Mass extinction coefficient• The density of the absorber is given by:ρ = M HAWhere M is the mass of absorber in the solution, H is the depth of thesolution, and A is the horizontal area of the ‘container’.• The transmittance is then:€t = exp(−τ ) = exp(−β eH)⎡⎡ ⎛⎛ M ⎞⎞ ⎤⎤= exp⎢⎢−k e ⎜⎜ ⎟⎟ H⎣⎣ ⎝⎝ HA⎠⎠⎥⎥⎦⎦= exp ⎡⎡−k ⎛⎛⎢⎢ e⎜⎜⎣⎣ ⎝⎝⇒ τ = k e• So the transmittance does not depend on H, only M and A.MAMA⎞⎞ ⎤⎤⎟⎟⎠⎠⎥⎥⎦⎦€Extinction cross-section• The dimensions of k e are area per unit mass, or extinction cross-section perunit mass• The extinction cross-section is used when considering molecules of anabsorbing gas, droplets of water in a cloud, or soot particles in a smokeplume.• We use the number density (N; m -3 ) to characterize the particleconcentration.• To link the extinction coefficient and N we can write:• Where the constant of proportionality σ e has dimensions of area and is theextinction cross-section; or the effective area of the particle ‘blocking’transmission of radiation€β e= σ eN15
ONREMOTE CONTROLTX IN RX IN PPS-CONT TX OUT RX OUTOFFACC-J1ACC-J2ACC-J4ACC-J3BAT+ -13.8VACRF OUTAMP-CONT110/220V13.8 VDCExternalPower SourceSeriesProtectionDiodeFuse30AFigure 7. RM125/RM125R Connections to DC Power SourceInstallation Procedure – Radio with PPS OptionPPS FunctionsThe optional Pre-Selector/Post-Selector, PPS-100, supported as an option by the RM125/RM125R,permits operation of collocated receivers and transmitters on frequencies separated by as little as10%. The PPS operating frequency range is 1.60 to 29.99 MHz.To use the PPS, order the RM125/RM125R with option G65. The connections between anRM125/RM125R with option G65 and the PPS are shown in Figure 8.When the G65 option is installed, the PPS is controlled by the RM125/RM125R through serial andparallel ports and is automatically inserted by the RM125/RM125R in the signal path in accordancewith the operating mode:• In the receive mode, the PPS functions as a preselector, providing an additional front endselectivity stage for the receive path of the RM125/RM125R. This reduces the receiverdesensitization and overload that would normally occur in the presence of strong adjacent RFtransmissions.• In the transmit mode, the PPS is used as a postselector. It attenuates spurious signals andbroadband noise in the driver transmit signal before it reaches the internal RF power amplifier,thereby reducing interference to neighboring receivers.In both modes, the PPS automatically tracks the RM125/RM125R operating frequency. Rapid tuningmakes the PPS suitable for Automatic Link Establishment (ALE) or Adaptive Applications.During the power-up self-test, the PPS automatically switches to the bypass mode: in this mode, RFsignals pass directly through the PPS, without filtering, and therefore it does not interfere with systemoperation. The PPS also switches to the bypass mode when the RM125/RM125R frequency is outsidethe PPS operating range, 1.60 to 29.99 MHz.In case excessive RF power is applied to the PPS, the PPS enters the RF overload protection mode: inthis mode, the PPS antenna port is disconnected, and the internal antenna input is short-circuited toground. This also protects the RM125/RM125R internal transceiver against overload.The PPS is housed in a 1U high (1.75”) case intended for 19” rack mounting. Standard operation isfrom 115 or 230 VAC, 50/60 Hz; the PPS can also be powered from 12 or 24 VDC sources.16 6888882V02
3/31/10Generalization to mixtures of components• Real particles in the atmosphere are complex, and many different gasesand aerosol types are present…β e= ∑ β e,i= ∑ ρ ik e,i= ∑σ e,iN iiii€The total extinction, scatteringand absorption coefficients for amixture of components = sum ofthe corresponding coefficients foreach individual componentPlane parallel approximation• The atmosphere is usually approximated as plane-parallel in atmospheric remotesensing (also called slab geometry)• Ignore horizontal variations and the curvature of the earthHcosθ
3/31/10Slant and vertical atmospheric pathsOptical depthz 2∫τ (z 1,z 2) =z 1β e(z) dzTransmittanceθ = solar zenith angle0º = overhead sun90º = sun at horizon€t = e − τ µNB. Optical depth does notdepend on the optical path,but the optical path andtransmittance do• Remote sensing is often done when the sun is not directly overhead, sothe radiation follows a slant path rather than a vertical path€Satellite viewing geometryµ is also referred to asthe (geometrical) airmass factor• Satellites measuring reflected and scattered radiation (UV/visible/SWIR)detect radiation that has been transmitted twice through the atmosphere18
3/31/10<strong>Transmission</strong> spectrum of the atmosphere• At which wavelengths is the cloud-free atmosphere relativelytransparent? (<strong>Atmospheric</strong> windows)• At which wavelengths is the cloud-free atmosphere strongly absorbing,and which constituents are responsible for the absorption?• How do the extinction and scattering properties of clouds vary withwavelength?Transmittance1Constituent<strong>Atmospheric</strong> compositionFraction (byvolume) indry airSignificantabsorption bandsNitrogen, N 2 78.1% -Oxygen, O 2 20.9% UV-C, MW near 60and 118 GHz, weak inVIS and IRH 2 O 0-2 % Numerous strongbands in IR; also MWnear 183 GHzRemarksHighly variable intime and spaceAr & inert gases 0.936% - monoatomicCO 2 370 ppm Near 2.8, 4.3, and 15µmIncreasing 1.6ppm/yearMethane, CH 4 1.7 ppm Near 3.3 and 7.8 µm IncreasingNitrous oxide, N 2 O 0.35 ppm 4.5, 7.8 and 17 µmCarbon monoxide, CO 0.07 ppm 4.7 µm (weak)Ozone, O 3 ~10 -8 UV-B, 9.6 µm19
3/31/10<strong>Atmospheric</strong> transmittanceUV-IR(no scattering)NB. Midlatitude summerWhich constituents have thelargest influence onatmospheric transmittance?Where are the atmosphericwindow regions?Which gases are ‘infraredactive’ and contribute togreenhouse warming?Which gases significantlyabsorb solar radiation?What are the main sourcesfor each gas?<strong>Atmospheric</strong> CO 2Measurements of atmospheric CO 2 concentrations at Mauna Loa Observatory, Hawaii(Keeling and Whorf, Scripps Inst. Ocean., UCA)20
3/31/10Microwave transmittanceTotal atmosphericwater vapor retrievalsNB. Midlatitude conditionsMicrowavespenetrate clouds!3 cm<strong>Atmospheric</strong>temperature retrievals1 mm<strong>Atmospheric</strong> humidityprofile retrievalsEffects of scatteringNB. Midlatitude summer conditionsScatteringThe scattering cross-section (σ s ) of an air molecule is proportional to λ -4How much stronger is scattering at 0.4 µm compared to 0.7 µm?21
3/31/10Extinction and scattering by aerosols and clouds2006 Annual Mean Aerosol Optical Depth (AOD) at 550 nm from MODISHigh Aerosol AOD:FiresVolcanic eruptionsDust stormsSevere pollutionExtinction and scattering by aerosols and cloudsWhere do clouds onlyscatter radiation (ω = 1)?Where could ice andwater clouds bedistinguished?22
3/31/10Measuring solar intensity from the ground• We know that the solar irradiance atthe top of the atmosphere (S 0 ) is ofcrucial importance to Earth’s radiationbudget• Satellite sensors now measure thesolar flux and solar spectrum, but howwas this done before the first satellites,using ground-based measurements?• Problem: solar intensity measured atground level is reduced byatmospheric absorption and scattering,but the atmospheric transmittance isunknown. But the latter cannot beinferred without knowing the solar flux– hence a ‘Catch 22’ situation.Measuring solar intensity from the ground• Solution:• Assume a plane-parallel atmosphere with constant properties overthe course of a day (i.e., a clear day with stable temperature,pressure)• For any given wavelength (λ), there are 2 unknowns: the solarintensity (S λ ) and the atmospheric optical depth (τ λ )• From Beer’s <strong>Law</strong>, the intensity of solar radiation measured at sealevel (I λ ) is:µ = cos θ and θ = solar zenith angle23
3/31/10Measuring solar intensity from the ground• Now take the logarithm of both sides:• Define x = 1/µ = sec θ then this is a linear equation of the form y = mx+c,where y = ln(I λ ), the slope m = -τ λ and the y-intercept c = ln(S λ ).• Hence to determine τ λ and S λ , measure y for a range of values of x, i.e. atdifferent times of day as the sun rises, reaches its zenith, and sets. Plot thedata and find the slope and y-intercept.(θ = 0º)Sun photometerhttp://aeronet.gsfc.nasa.gov/Global network of sun photometers measuring aerosol optical depthCalibrated using Langley technique at Mauna Loa Observatory, Hawaii24
3/31/10The exponential atmosphere• The density of the atmosphere decays exponentially with height z:ρ(z) = ρ 0e − z H• Where ρ 0 is the density at sea level and H (≈ 8 km) is the scale height (thealtitude change that leads to a factor e change in density)• So for a ‘well-mixed’ constituent (like CO 2 ), its density is:€• where w 1 is the mixing ratio (mass of constituent per unit mass of air)• Assume a mass absorption coefficient k a for the constituent that dependson λ but not T or P, and a nonscattering atmosphere at the λ of interest:€ρ 1(z) = w 1ρ 0e − z Hβ e(z) = k aw 1ρ 0e − z H€Optical depth in an exponential atmosphereτ (z) =∞∞∫ β e( z ʹ′ )dz ʹ′ = k aw 1ρ 0 ∫ e − ʹ′zzz Hdzʹ′τ (z) = k aw 1ρ 0He − z H€Optical depth between altitude z andthe top-of-the-atmosphere (TOA)€τ* = τ (0) = k aw 1ρ 0HTOTAL <strong>Atmospheric</strong> Optical Depth€Optical depth increases more rapidlytowards the surface25
3/31/10Mass pathτ (z) = k aw 1ρ 0∞∫ze − z ʹ′Hdz ʹ′ = k au(z)€€u(z) =∞∫zρ 1( z ʹ′ )dzʹ′• Mass path of the constituentbetween altitude z and the top-ofthe-atmosphere(TOA)• Dimensions: mass per unit area(e.g., g m -2 )• Integral over all z for water vaporgives an important atmosphericquantity: total precipitable water,water vapor burden, water vaporpath, liquid water path, cloud waterpath….(also for ice)Ice Water Path (IWP)Transmittance in an exponential atmosphere⎡⎡t(z) = exp − τ (z) ⎤⎤ ⎡⎡⎢⎢ ⎥⎥ = exp − k w a 1ρ 0H⎢⎢⎣⎣ µ ⎦⎦ ⎣⎣ µe − z H⎤⎤⎥⎥⎦⎦€Where is the absorption occurring?low atmospheric densityNo penetration of radiationt€W(z) = dt(z)dz= β e(z)µ t(z)The local rate of absorption withinthe atmosphere (W(z)) equals thelocal rate of change of transmittancefrom level z to the TOA, or the localextinction coefficient at level z timesthe transmittance from z to the TOAW(z) is called the absorptionweighting function. Its peak dependson k e at the wavelength in question,but occurs where τ(z) = 126
3/31/10Optical thickness and transmittance of cloudsCan you see the sun through a cloud? a (absorptance)r (reflectance or albedo)Liquid water cloudsDroplet radii: 5-15 µmN = 10 2 -10 3 cm -3t dir + t diff + r + a = 1t + r + a = 1t = t dir + t difft diff (diffuse transmittance)t dir (direct transmittance)r = reflected to spacet = available for surface heatinga = heats atmosphere locallyOptical thickness and transmittance of clouds• t dir , t diff , r and a depend on the cloud optical thickness (τ*), single-scatteralbedo (ω) and how radiation is scattered by the cloud droplets• Values of t diff , r and a depend on multiple scattering of radiation by thecloud droplets• Consider a monodisperse cloud composed of droplets of identical radius(r) with a concentration N m -3• Volume extinction coefficient:β e= NQ eπr 2€27
3/31/10Optical thickness and transmittance of clouds• Note that N and r are difficult to measure, but we can measure or estimatethe cloud water density ρ w (typically 0.1-1 g m -3 )• For a monodisperse cloud, the cloud water density is N times the mass ofwater in each droplet:ρ w= N 4 3 πr 3 ρ l• where ρ l is the density of pure water (~1000 kg m -3 )• This gives us a new expression for the volume extinction coefficient:€ β e= NQ eπr 2 = k eρ w= k eN 4 3 πr 3 ρ l€• And solving for k e gives:k e= 3Q e4ρ lrNote inversedependence on r!€Rain and fogr ≈ 1 mmk e = 1.5 m 2 kg -1ρ w = 0.1 g m -3Q e ≈ 2 at visible wavelengthsk e= 3Q e4ρ lrr ≈ 10 µmk e = 150 m 2 kg -1ρ w = 0.1 g m -3β e = 0.15 km -1β e= k eρ wβ e = 15 km -1€€28
3/31/10Indirect Effect in Nature (from MODIS)What are these?MODIS visible image; Atlantic coast of Europe; January 27, 2003Ship TracksShip Ship Exhaust CDNC = Cloud Droplet Number Concentration(# cloud condensation nuclei)⎡⎡ 9πL 2 Hτ* = Q e ⎢⎢ 2⎣⎣ 16ρ l⎤⎤N⎥⎥⎦⎦13L = liquid water path, H = geometric cloud thicknessi.e. Optical thickness proportional to N 1/3€29
3/31/10The Aerosol Indirect Effect• The impact of aerosols on cloud properties (and henceclimate) is called the aerosol indirect effect• A high concentration of aerosols ‘overseed’ cloud droplets togenerate highly concentrated, narrowly distributed clouddroplet spectra• This can increase the cloud albedo by up to 30%, reducingthe amount of radiation reaching the surface• Narrowly distributed cloud droplet spectra prevent theformation of precipitation and could increase cloud lifetimethat further cools the Earth’s surface• Contrast with the aerosol direct effectSmoke inhibitscloud formationover the AmazonMODIS visible imageAmazon; August 11, 2002[Koren et al., Science, 2004]30
3/31/10MODIS visible imageGreat LakesPolydisperse clouds• The assumption of a single droplet size (monodisperse) is not realistic• Real clouds are polydisperse and contain a range of droplet sizesdescribed by a drop size distribution n(r)• n(r) drNumber of droplets (per unit volume of air)whose radii fall in the range [r, r + dr]€€€• Dimensions are length -4 ; e.g., m -3 µm -1N =A sfc=β e=∞∫0∞∫0n(r) dr∞∫0n(r)[4πr 2 ]drn(r)[Q e(r)πr 2 ]drTotal number densityTotal surface areaExtinction coefficient€r eff=∞∫0∞∫0n(r)r 3 drn(r)r 2 dr‘Effective’ radius31