Columns for HPLC - Western Analytical
Columns for HPLC - Western Analytical
Columns for HPLC - Western Analytical
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
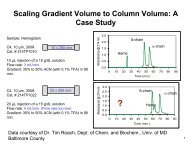
<strong>Columns</strong> <strong>for</strong> <strong>HPLC</strong><strong>Columns</strong> <strong>for</strong> <strong>HPLC</strong><strong>Analytical</strong> columns packed with NUCLEOSIL ®Unmodified NUCLEOSIL ® , polar NUCLEOSIL ® phases and ion exchangersPackings with chemically bonded polar phasesThese materials are characterised by fast mass transfer, becausethe bonded organic layer of these stationary phases ismonomeric, thus minimising diffusion paths.Due to the availability of different functional groups it is possibleto vary the chemical characteristics of the surface andconsequently the adsorption characteristics of the stationaryphase. Silica packings with bonded polar phases thus combinethe advantages of partition chromatography (variation ofadsorption properties) and adsorption chromatography (rapidmass transfer in the stationary phase). Further advantagesof chemically bonded polar phases are:They do not “bleed”; equilibration with a liquid stationaryphase is not necessary as is the case in classical partitionchromatography.The influence of impurities in the eluent (e. g. water) onthe separation qualities of bonded polar phases is muchsmaller than is the case with unmodified silica gels andaluminium oxides <strong>for</strong> adsorption chromatography.Swelling and consequently variation in the permeabilityof the packed columns never occurs with chemicallybonded polar silica phases. Even the use of water orother aqueous eluents does not present any problems.Although the chemically bonded phases on the surface orthe silica gel <strong>for</strong>m a monomeric organic layer, they have alarge surface concentration of functional groups, which isabout 3 to 4 functional groups per 100 Å 2 , i. e. about5 µeq/m 2 .Influence of functional groups on the separation characteristics of a stationary phasek’10,09,08,07,06,0Effect of modification on the separationof aromatic hydrocarbonsNO 2 phaseThe figure on the left shows the influence of the modificationof silica gel on the k’ values (capacity factors) of aromatic hydrocarbons,with n-heptane as eluent on unmodified NUCL-EOSIL ® as well as three chemically bonded polar phases,namely NUCLEOSIL ® CN, NH 2 and NO 2 . On the y-axis thek’ values of 9 aromatic hydrocarbons have been plotted. Theinfluence of the nitroaromatic groups on the separation is especiallyevident. A strong interaction between the electronicsystems results in the <strong>for</strong>mation of charge transfer complexeswith considerably larger k’ values than <strong>for</strong> unmodifiedNUCLEOSIL ® .5,0NH 2 phase4,03,02,0NUCLEOSILunmodified1,00BenzeneNaphthaleneBiphenylAnthracenePhenanthrenePyreneChryseneBenzo[a]pyreneCN phaseBenzo[e]pyrene6.7.05MNwww.mn-net.com49