Fluorescence
Fluorescence
Fluorescence
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
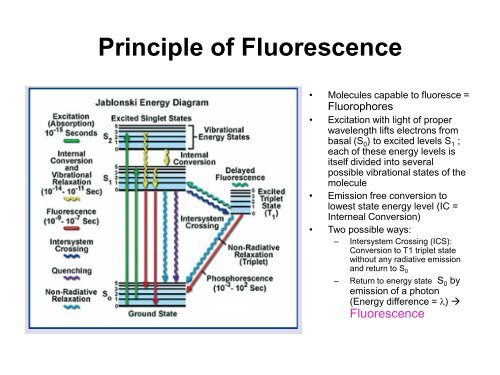
Principle of <strong>Fluorescence</strong>• Molecules capable to fluoresce =Fluorophores• Excitation with light of properwavelength lifts electrons frombasal (S 0 ) to excited levels S 1 ;each of these energy levels isitself divided into severalpossible vibrational states of themolecule• Emission free conversion tolowest state energy level (IC =Interneal Conversion)• Two possible ways:– Intersystem Crossing (ICS):Conversion to T1 triplet statewithout any radiative emissionand return to S 0– Return to energy state S 0 byemission of a photon(Energy difference = l) <strong>Fluorescence</strong>