Collaborative Intravenous Nursing Service (CINS) Guidelines
Collaborative Intravenous Nursing Service (CINS) Guidelines
Collaborative Intravenous Nursing Service (CINS) Guidelines
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
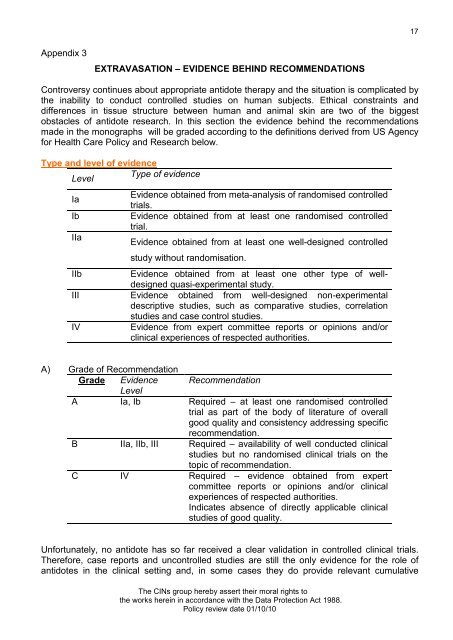
17Appendix 3EXTRAVASATION – EVIDENCE BEHIND RECOMMENDATIONSControversy continues about appropriate antidote therapy and the situation is complicated bythe inability to conduct controlled studies on human subjects. Ethical constraints anddifferences in tissue structure between human and animal skin are two of the biggestobstacles of antidote research. In this section the evidence behind the recommendationsmade in the monographs will be graded according to the definitions derived from US Agencyfor Health Care Policy and Research below.Type and level of evidenceLevelType of evidenceIaIbIIaEvidence obtained from meta-analysis of randomised controlledtrials.Evidence obtained from at least one randomised controlledtrial.Evidence obtained from at least one well-designed controlledstudy without randomisation.IIbEvidence obtained from at least one other type of welldesignedquasi-experimental study.III Evidence obtained from well-designed non-experimentaldescriptive studies, such as comparative studies, correlationstudies and case control studies.IVEvidence from expert committee reports or opinions and/orclinical experiences of respected authorities.A) Grade of RecommendationGrade Evidence RecommendationLevelA Ia, Ib Required – at least one randomised controlledtrial as part of the body of literature of overallgood quality and consistency addressing specificrecommendation.B IIa, IIb, III Required – availability of well conducted clinicalstudies but no randomised clinical trials on thetopic of recommendation.C IV Required – evidence obtained from expertcommittee reports or opinions and/or clinicalexperiences of respected authorities.Indicates absence of directly applicable clinicalstudies of good quality.Unfortunately, no antidote has so far received a clear validation in controlled clinical trials.Therefore, case reports and uncontrolled studies are still the only evidence for the role ofantidotes in the clinical setting and, in some cases they do provide relevant cumulativeThe CINs group hereby assert their moral rights tothe works herein in accordance with the Data Protection Act 1988.Policy review date 01/10/10