View - ResearchGate
View - ResearchGate
View - ResearchGate
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
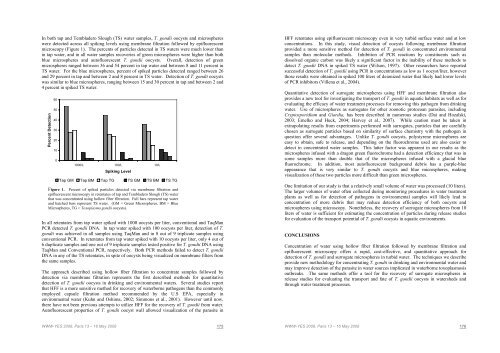
In both tap and Tembladero Slough (TS) water samples, T. gondii oocysts and microsphereswere detected across all spiking levels using membrane filtration followed by epifluorescentmicroscopy (Figure 1). The percents of particles detected in TS waters were much lower thanin tap water, and in all water samples recoveries of green microspheres were higher than bothblue microspheres and autofluorescent T. gondii oocysts. Overall, detection of greenmicrospheres ranged between 36 and 54 percent in tap water and between 8 and 11 percent inTS water. For the blue microspheres, percent of spiked particles detected ranged between 26and 29 percent in tap and between 2 and 8 percent in TS water. Detection of T. gondii oocystswas similar to blue microspheres, ranging between 15 and 30 percent in tap and between 2 and4 percent in spiked TS water.Percent Detection60504030201001000/L 100/L 10/LSpiking LevelTap GM Tap BM Tap TG TS GM TS BM TS TGFigure 1. Percent of spiked particles detected via membrane filtration andepifluorescent microscopy in retentates of tap and Tembladero Slough (TS) waterthat was concentrated using hollow fiber filtration. Full bars represent tap waterand hatched bars represent TS water. (GM = Green Microspheres, BM = BlueMicrospheres, TG = Toxoplasma gondii oocysts).In all retentates from tap water spiked with 1000 oocysts per liter, conventional and TaqManPCR detected T. gondii DNA. In tap water spiked with 100 oocysts per liter, detection of T.gondii was achieved in all samples using TaqMan and in 8 out of 9 triplicate samples usingconventional PCR. In retentates from tap water spiked with 10 oocysts per liter, only 4 out of6 duplicate samples and one out of 9 triplicate samples tested positive for T. gondii DNA usingTaqMan and Conventional PCR, respectively. Both PCR methods failed to detect T. gondiiDNA in any of the TS retentates, in spite of oocysts being visualized on membrane filters fromthe same samples.The approach described using hollow fiber filtration to concentrate samples followed bydetection via membrane filtration represents the first described methods for quantitativedetection of T. gondii oocysts in drinking and environmental waters. Several studies reportthat HFF is a more sensitive method for recovery of waterborne pathogens than the commonlyemployed capsule filtration method recommended by the U.S EPA, especially inenvironmental water (Kuhn and Oshima, 2002; Simmons et al., 2001). However until now,there have not been previous attempts to utilize HFF for the recovery of T. gondii from water.Autofluorescent properties of T. gondii oocyst wall allowed visualization of the parasite inHFF retentates using epifluorescent microscopy even in very turbid surface water and at lowconcentrations. In this study, visual detection of oocysts following membrane filtrationprovided a more sensitive method for detection of T. gondii in concentrated environmentalsamples than molecular methods. Inhibition of PCR reactions by constituents such asdissolved organic carbon was likely a significant factor in the inability of these methods todetect T. gondii DNA in spiked TS water (Wilson, 1997). Other researchers have reportedsuccessful detection of T. gondii using PCR in concentrations as low as 1 oocyst/liter, howeverthose results were obtained in spiked 100 liters of deionized water that likely had lower levelsof PCR inhibitors (Villena et al., 2004).Quantitative detection of surrogate microspheres using HFF and membrane filtration alsoprovides a new tool for investigating the transport of T. gondii in aquatic habitats as well as forevaluating the efficacy of water treatment processes for removing this pathogen from drinkingwater. Use of microspheres as surrogates for other zoonotic protozoan parasites, includingCryptosporidium and Giardia, has been described in numerous studies (Dai and Hozalski,2003; Emelko and Huck, 2004; Harvey et al., 2007). While caution must be taken inextrapolating results from experiments performed with surrogates, particles that are carefullychosen as surrogate particles based on similarity of surface chemistry with the pathogen inquestion offer several advantages. Unlike T. gondii oocysts, polystyrene microspheres areeasy to obtain, safe to release, and depending on the fluorochrome used are also easier todetect in concentrated water samples. This latter factor was apparent in our results as themicrospheres infused with a dragon green fluorochrome had a detection efficiency that was insome samples more than double that of the microspheres infused with a glacial bluefluorochrome. In addition, most autofluorescent background debris has a purple-blueappearance that is very similar to T. gondii oocysts and blue microspheres, makingvisualization of these two particles more difficult than green microspheres.One limitation of our study is that a relatively small volume of water was processed (10 liters).The larger volumes of water often collected during monitoring procedures in water treatmentplants as well as for detection of pathogens in environmental samples will likely lead toconcentration of more debris that may reduce detection efficiency of both oocysts andmicrospheres using microscopy. Nonetheless, the recovery of surrogate microspheres from 10liters of water is sufficient for estimating the concentration of particles during release studiesfor evaluation of the transport potential of T. gondii oocysts in aquatic environments.CONCLUSIONSConcentration of water using hollow fiber filtration followed by membrane filtration andepifluorescent microscopy offers a rapid, cost-effective, and quantitative approach fordetection of T. gondii and surrogate microspheres in turbid water. The techniques we describeprovide new methodology for concentrating T. gondii in drinking and environmental water andmay improve detection of the parasite in water sources implicated in waterborne toxoplasmosisoutbreaks. The same methods offer a tool for the recovery of surrogate microspheres inrelease studies for evaluating the transport and fate of T. gondii oocysts in watersheds andthrough water treatment processes.WWW-YES 2008, Paris 13 – 16 May 2008 175WWW-YES 2008, Paris 13 – 16 May 2008 176



![[pastel-00730831, v1] Incidence des pratiques d'entretien ... - LEESU](https://img.yumpu.com/50938896/1/184x260/pastel-00730831-v1-incidence-des-pratiques-dentretien-leesu.jpg?quality=85)











