Peptidoglycan .Types of Bacterial Cell Walls and their Taxonomic ...
Peptidoglycan .Types of Bacterial Cell Walls and their Taxonomic ...
Peptidoglycan .Types of Bacterial Cell Walls and their Taxonomic ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
VOLX 36, 1972<br />
PEPTIDOGLYCAN TYPES OF BACTERIAL CELL WALLS<br />
./*-* Mur<br />
x Glu<br />
-aLys<br />
E /-*--: 0----L-Ala-L-Ala<br />
°=°<br />
c . / ; o-- o L-Ala-D-Glu<br />
o1 - N6 -L-Ala-L-Lys<br />
ur 1.0<br />
U~~~~~~<br />
C<br />
.0<br />
zto.s .0. '°" \a<br />
. D-Ala-L-Ala<br />
-o0y D-Glu-L-Lys<br />
1 2 3 4 5hrs<br />
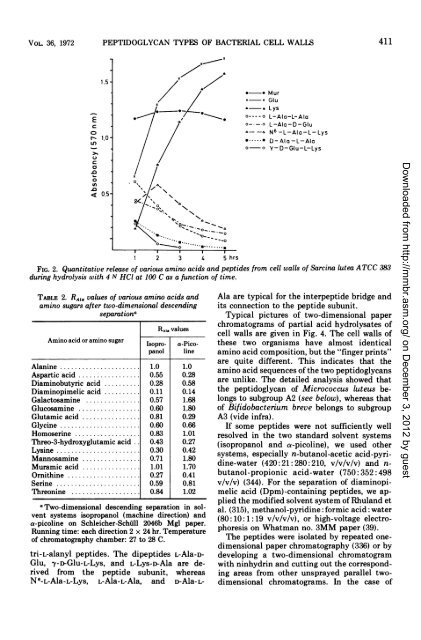
FIG. 2. Quantitative release <strong>of</strong> various amino acids <strong>and</strong> peptides from cell walls <strong>of</strong> Sarcina lutea ATCC 383<br />
during hydrolysis with 4 N HCI at 100 C as a function <strong>of</strong> time.<br />
TABLE 2. RAIG values <strong>of</strong> various amino acids <strong>and</strong><br />
amino sugars after two-dimensional descending<br />
separationa<br />
RAI. values<br />
Amino acid or amino sugar Isopro- a-Picopanol<br />
line<br />
Alanine ...................... 1.0 1.0<br />
Aspartic acid ................. 0.55 0.28<br />
Diaminobutyric acid .......... 0.28 0.58<br />
Diaminopimelic acid .......... 0.11 0.14<br />
Galactosamine ............... 0.57 1.68<br />
Glucosamine ................. 0.60 1.80<br />
Glutamic acid ................ 0.81 0.29<br />
Glycine ...................... 0.60 0.66<br />
Homoserine .................. 0.83 1.01<br />
Threo-3-hydroxyglutamic acid 0.43 0.27<br />
Lysine ....................... 0.30 0.42<br />
Mannosamine ................ 0.71 1.80<br />
Muramic acid ................ 1.01 1.70<br />
Ornithine .................... 0.27 0.41<br />
Serine ...................... 0.59 0.81<br />
Threonine ................... 0.84 1.02<br />
aTwo-dimensional descending separation in solvent<br />
systems isopropanol (machine direction) <strong>and</strong><br />
a-picoline on Schleicher-Schiill 2046b Mgl paper.<br />
Running time: each direction 2 x 24 hr. Temperature<br />
<strong>of</strong> chromatography chamber: 27 to 28 C.<br />
tri-L-alanyl peptides. The dipeptides L-Ala-D-<br />
Glu, y->-Glu-L-Lys, <strong>and</strong> L-Lys-D-Ala are derived<br />
from the peptide subunit, whereas<br />
N6-L-Ala-L-Lys, L-Ala-L-Ala, <strong>and</strong> D-Ala-L-<br />
411<br />
Ala are typical for the interpeptide bridge <strong>and</strong><br />
its connection to the peptide subunit.<br />
Typical pictures <strong>of</strong> two-dimensional paper<br />
chromatograms <strong>of</strong> partial acid hydrolysates <strong>of</strong><br />
cell walls are given in Fig. 4. The cell walls <strong>of</strong><br />
these two organisms have almost identical<br />
amino acid composition, but the "finger prints"<br />
are quite different. This indicates that the<br />
amino acid sequences <strong>of</strong> the two peptidoglycans<br />
are unlike. The detailed analysis showed that<br />
the peptidoglycan <strong>of</strong> Micrococcus luteus belongs<br />
to subgroup A2 (see below), whereas that<br />
<strong>of</strong> Bifidobacterium breve belongs to subgroup<br />
A3 (vide infra).<br />
If some peptides were not sufficiently well<br />
resolved in the two st<strong>and</strong>ard solvent systems<br />
(isopropanol <strong>and</strong> a-picoline), we used other<br />
systems, especially n-butanol-acetic acid-pyridine-water<br />
(420:21:280:210, v/v/v/v) <strong>and</strong> nbutanol-propionic<br />
acid-water (750: 352:498<br />
v/v/v) (344). For the separation <strong>of</strong> diaminopimelic<br />
acid (Dpm)-containing peptides, we applied<br />
the modified solvent system <strong>of</strong> Rhul<strong>and</strong> et<br />
al. (315), methanol-pyridine: formic acid: water<br />
(80:10: 1: 19 v/v/v/v), or high-voltage electrophoresis<br />
on Whatman no. 3MM paper (39).<br />
The peptides were isolated by repeated onedimensional<br />
paper chromatography (336) or by<br />
developing a two-dimensional chromatogram<br />
with ninhydrin <strong>and</strong> cutting out the corresponding<br />
areas from other unsprayed parallel twodimensional<br />
chromatograms. In the case <strong>of</strong><br />
Downloaded from<br />
http://mmbr.asm.org/<br />
on December 3, 2012 by guest