Hydrogen and its competitors, 2004
Hydrogen and its competitors, 2004
Hydrogen and its competitors, 2004
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
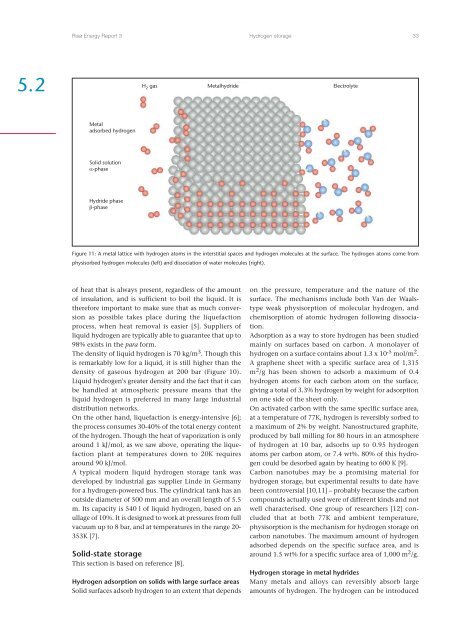
Risø Energy Report 3<strong>Hydrogen</strong> storage 335.2H 2 gas Metalhydride ElectrolyteMetaladsorbed hydrogenSolid solutionα-phaseHydride phaseβ-phaseFigure 11: A metal lattice with hydrogen atoms in the interstitial spaces <strong>and</strong> hydrogen molecules at the surface. The hydrogen atoms come fromphysisorbed hydrogen molecules (left) <strong>and</strong> dissociation of water molecules (right).of heat that is always present, regardless of the amountof insulation, <strong>and</strong> is sufficient to boil the liquid. It istherefore important to make sure that as much conversionas possible takes place during the liquefactionprocess, when heat removal is easier [5]. Suppliers ofliquid hydrogen are typically able to guarantee that up to98% exists in the para form.The density of liquid hydrogen is 70 kg/m 3 . Though thisis remarkably low for a liquid, it is still higher than thedensity of gaseous hydrogen at 200 bar (Figure 10).Liquid hydrogen's greater density <strong>and</strong> the fact that it canbe h<strong>and</strong>led at atmospheric pressure means that theliquid hydrogen is preferred in many large industrialdistribution networks.On the other h<strong>and</strong>, liquefaction is energy-intensive [6];the process consumes 30-40% of the total energy contentof the hydrogen. Though the heat of vaporization is onlyaround 1 kJ/mol, as we saw above, operating the liquefactionplant at temperatures down to 20K requiresaround 90 kJ/mol.A typical modern liquid hydrogen storage tank wasdeveloped by industrial gas supplier Linde in Germanyfor a hydrogen-powered bus. The cylindrical tank has anoutside diameter of 500 mm <strong>and</strong> an overall length of 5.5m. Its capacity is 540 l of liquid hydrogen, based on anullage of 10%. It is designed to work at pressures from fullvacuum up to 8 bar, <strong>and</strong> at temperatures in the range 20-353K [7].Solid-state storageThis section is based on reference [8].<strong>Hydrogen</strong> adsorption on solids with large surface areasSolid surfaces adsorb hydrogen to an extent that dependson the pressure, temperature <strong>and</strong> the nature of thesurface. The mechanisms include both Van der Waalstypeweak physisorption of molecular hydrogen, <strong>and</strong>chemisorption of atomic hydrogen following dissociation.Adsorption as a way to store hydrogen has been studiedmainly on surfaces based on carbon. A monolayer ofhydrogen on a surface contains about 1.3 x 10 -5 mol/m 2 .A graphene sheet with a specific surface area of 1,315m 2 /g has been shown to adsorb a maximum of 0.4hydrogen atoms for each carbon atom on the surface,giving a total of 3.3% hydrogen by weight for adsorptionon one side of the sheet only.On activated carbon with the same specific surface area,at a temperature of 77K, hydrogen is reversibly sorbed toa maximum of 2% by weight. Nanostructured graphite,produced by ball milling for 80 hours in an atmosphereof hydrogen at 10 bar, adsorbs up to 0.95 hydrogenatoms per carbon atom, or 7.4 wt%. 80% of this hydrogencould be desorbed again by heating to 600 K [9].Carbon nanotubes may be a promising material forhydrogen storage, but experimental results to date havebeen controversial [10,11] – probably because the carboncompounds actually used were of different kinds <strong>and</strong> notwell characterised. One group of researchers [12] concludedthat at both 77K <strong>and</strong> ambient temperature,physisorption is the mechanism for hydrogen storage oncarbon nanotubes. The maximum amount of hydrogenadsorbed depends on the specific surface area, <strong>and</strong> isaround 1.5 wt% for a specific surface area of 1,000 m 2 /g.<strong>Hydrogen</strong> storage in metal hydridesMany metals <strong>and</strong> alloys can reversibly absorb largeamounts of hydrogen. The hydrogen can be introduced