Chemistry_Today_April_2017_vk_com_stopthepress
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
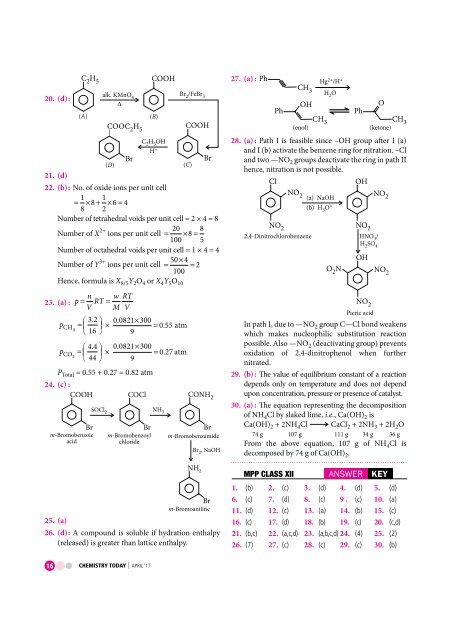
20. (d) :<br />
CH 2 5<br />
alk. KMnO 4<br />
( A)<br />
COOC H 2 5<br />
( D)<br />
<br />
Br<br />
COOH<br />
( B)<br />
CHOH 2 5<br />
H +<br />
Br 2/FeBr3<br />
COOH<br />
21. (d)<br />
22. (b): No. of oxide ions per unit cell<br />
1<br />
= × + × =<br />
8 8 1 2 6 4<br />
Number of tetrahedral voids per unit cell = 2 × 4 = 8<br />
Number of X 2+ 20<br />
ions per unit cell = × =<br />
100 8 8<br />
5<br />
Number of octahedral voids per unit cell = 1 × 4 = 4<br />
Number of Y 3+ 50× 4<br />
ions per unit cell = = 2<br />
100<br />
Hence, formula is X 8/5 Y 2 O 4 or X 4 Y 5 O 10<br />
n<br />
23. (a) : p = V RT = w RT<br />
M V<br />
32<br />
p CH4 = . 0.<br />
0821×<br />
300<br />
×<br />
16 9<br />
( C)<br />
= 055 . atm<br />
44<br />
p CO2 = . 0.<br />
0821×<br />
300<br />
× = 027 . atm<br />
44 9<br />
P Total = 0.55 + 0.27 = 0.82 atm<br />
24. (c) :<br />
COOH<br />
COCl<br />
CONH 2<br />
Br<br />
m-Bromobenzoic<br />
acid<br />
SOCl 2<br />
Br<br />
m-Bromobenzoyl<br />
chloride<br />
NH 3<br />
Br<br />
Br<br />
m-Bromobenzamide<br />
Br , NaOH<br />
2<br />
27. (a) : Ph<br />
Ph<br />
CH 3<br />
OH<br />
(enol)<br />
Hg 2+ /H+<br />
HO 2<br />
CH 3<br />
Ph<br />
O<br />
CH 3<br />
(etone) k<br />
28. (a) : Path I is feasible since –OH group after I (a)<br />
and I (b) activate the benzene ring for nitration. –Cl<br />
and two —NO 2 groups deactivate the ring in path II<br />
hence, nitration is not possible.<br />
Cl<br />
OH<br />
NO 2<br />
NO 2 (a) NaOH<br />
NO 2<br />
2,4-Dinitrochlorobenzene<br />
(b) HO+<br />
3<br />
NO 2<br />
HNO / 3<br />
HSO 2 4<br />
OH<br />
ON 2 NO 2<br />
NO 2<br />
Picric acid<br />
In path I, due to —NO 2 group C—Cl bond weakens<br />
which makes nucleophilic substitution reaction<br />
possible. Also —NO 2 (deactivating group) prevents<br />
oxidation of 2,4-dinitrophenol when further<br />
nitrated.<br />
29. (b) : The value of equilibrium constant of a reaction<br />
depends only on temperature and does not depend<br />
upon concentration, pressure or presence of catalyst.<br />
30. (a) : The equation representing the de<strong>com</strong>position<br />
of NH 4 Cl by slaked lime, i.e., Ca(OH) 2 is<br />
Ca(OH) 2 + 2NH 4 Cl CaCl 2 + 2NH 3 + 2H 2 O<br />
74 g 107 g 111 g 34 g 36 g<br />
From the above equation, 107 g of NH 4 Cl is<br />
de<strong>com</strong>posed by 74 g of Ca(OH) 2 .<br />
NH 2<br />
Br<br />
m-Bromoaniline<br />
25. (a)<br />
26. (d): A <strong>com</strong>pound is soluble if hydration enthalpy<br />
(released) is greater than lattice enthalpy.<br />
MPP CLASS XII<br />
ANSWER<br />
KEY<br />
1. (b) 2. (c) 3. (d) 4. (d) 5. (d)<br />
6. (c) 7. (d) 8. (c) 9 . (c) 10. (a)<br />
11. (d) 12. (c) 13. (a) 14. (b) 15. (c)<br />
16. (c) 17. (d) 18. (b) 19. (c) 20. (c,d)<br />
21. (b,c) 22. (a,c,d) 23. (a,b,c,d) 24. (4) 25. (2)<br />
26. (7) 27. (c) 28. (c) 29. (c) 30. (b)<br />
16 CHEMISTRY TODAY | APRIL ‘17