Chemistry_Today_April_2017_vk_com_stopthepress
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
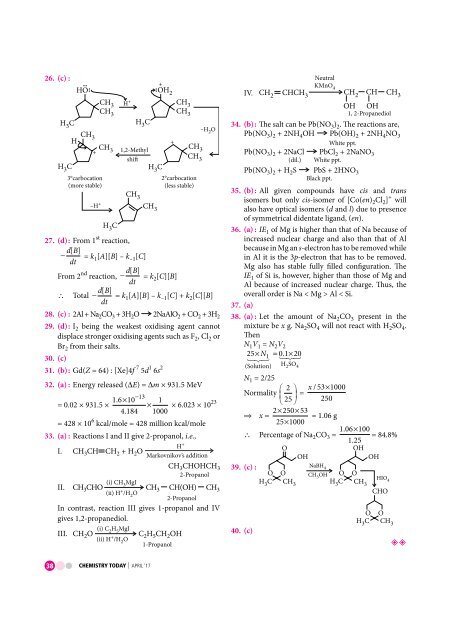
26. (c) :<br />
HC 3<br />
HC 3<br />
–H O<br />
CH 2<br />
3<br />
H<br />
+<br />
CH CH<br />
+ 3 1,2-Methyl<br />
3<br />
shi<br />
CH 3<br />
HC 3 HC 3<br />
HO:<br />
: OH 2<br />
CH 3 H +<br />
CH 3<br />
CH 3<br />
CH 3<br />
3°carbocation<br />
2°carbocation<br />
–H + CH 3<br />
(more stable)<br />
(less stable)<br />
CH 3<br />
:<br />
HC 3<br />
27. (d): From 1 st reaction,<br />
− dB [ ] = k1 [A][B] – k<br />
dt<br />
–1 [C]<br />
From 2 nd reaction, − dB [ ] = k2 [C][B]<br />
dt<br />
∴ Total − dB [ ] = k1 [A][B] – k –1 [C] + k 2 [C][B]<br />
dt<br />
28. (c) : 2Al + Na 2 CO 3 + 3H 2 O 2NaAlO 2 + CO 2 + 3H 2<br />
29. (d) : I 2 being the weakest oxidising agent cannot<br />
displace stronger oxidising agents such as F 2 , Cl 2 or<br />
Br 2 from their salts.<br />
30. (c)<br />
31. (b): Gd(Z = 64) : [Xe]4f 7 5d 1 6s 2<br />
32. (a) : Energy released (∆E) = ∆m × 931.5 MeV<br />
−13<br />
16 . × 10 1<br />
= 0.02 × 931.5 × × × 6.023 × 10 23<br />
4.<br />
184 1000<br />
= 428 × 10 6 kcal/mole = 428 million kcal/mole<br />
33. (a) : Reactions I and II give 2-propanol, i.e.,<br />
I. CH 3 CH<br />
H<br />
CH 2 + H 2 O<br />
Markovnikov’s addition<br />
CH 3 CHOHCH 3<br />
2-Propanol<br />
(i) CH3MgI<br />
II. CH 3 CHO CH<br />
+<br />
3 CH(OH)<br />
(ii) H /HO 2<br />
2-Propanol<br />
CH 3<br />
In contrast, reaction III gives 1-propanol and IV<br />
gives 1,2-propanediol.<br />
III. CH 2 O<br />
(i) C2HMgI<br />
5<br />
(ii) H + /HO 2<br />
C 2 H 5 CH 2 OH<br />
1-Propanol<br />
Neutral<br />
KMnO 4<br />
IV. <br />
<br />
<br />
<br />
O O<br />
1, 2-Propanediol<br />
34. (b): The salt can be Pb(NO 3 ) 2 . The reactions are,<br />
Pb(NO 3 ) 2 + 2NH 4 OH Pb(OH) 2 + 2NH 4 NO 3<br />
White ppt.<br />
Pb(NO 3 ) 2 + 2NaCl PbCl 2 + 2NaNO 3<br />
(dil.) White ppt.<br />
Pb(NO 3 ) 2 + H 2 S PbS + 2HNO 3<br />
Black ppt.<br />
35. (b): All given <strong>com</strong>pounds have cis and trans<br />
isomers but only cis-isomer of [Co(en) 2 Cl 2 ] + will<br />
also have optical isomers (d and l) due to presence<br />
of symmetrical didentate ligand, (en).<br />
36. (a) : IE 1 of Mg is higher than that of Na because of<br />
increased nuclear charge and also than that of Al<br />
because in Mg an s-electron has to be removed while<br />
in Al it is the 3p-electron that has to be removed.<br />
Mg also has stable fully filled configuration. The<br />
IE 1 of Si is, however, higher than those of Mg and<br />
Al because of increased nuclear charge. Thus, the<br />
overall order is Na < Mg > Al < Si.<br />
37. (a)<br />
38. (a) : Let the amount of Na 2 CO 3 present in the<br />
mixture be x g. Na 2 SO 4 will not react with H 2 SO 4 .<br />
Then<br />
N 1 V 1 = N 2 V 2<br />
25× N1<br />
= 01 . × 20<br />
<br />
( Solution)<br />
HSO 2 4<br />
N 1 = 2/25<br />
2 x /53×<br />
1000<br />
Normality <br />
25<br />
=<br />
250<br />
2× 250×<br />
53<br />
⇒ x = = 1.06 g<br />
25×<br />
1000<br />
106 . × 100<br />
∴ Percentage of Na 2 CO 3 = = 84.8%<br />
125 .<br />
O<br />
OH<br />
OH<br />
OH<br />
39. (c) :<br />
NaBH 4<br />
O O CH O O<br />
3OH<br />
HIO<br />
HC 3 CH 3 HC CH 4<br />
3 3<br />
CHO<br />
O O<br />
HC 3 CH 3<br />
40. (c)<br />
<br />
38 CHEMISTRY TODAY | APRIL ‘17