Chemistry_Today_April_2017_vk_com_stopthepress
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
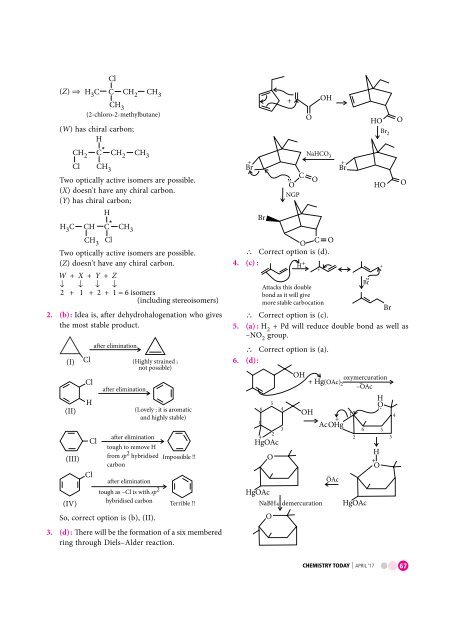
(Z) ⇒<br />
+<br />
OH<br />
(W) has chiral carbon;<br />
Two optically active isomers are possible.<br />
(X) doesn't have any chiral carbon.<br />
(Y) has chiral carbon;<br />
Br<br />
+ C<br />
– ..<br />
O<br />
NGP<br />
O<br />
HO O<br />
NaHCO 3<br />
Br 2<br />
O<br />
Br<br />
+<br />
HO<br />
O<br />
Br<br />
Two optically active isomers are possible.<br />
(Z) doesn't have any chiral carbon.<br />
2. (b) : Idea is, after dehydrohalogenation who gives<br />
the most stable product.<br />
Cl<br />
after elimination<br />
(I) (Highly strained ;<br />
not possible)<br />
(II)<br />
Cl<br />
H<br />
after elimination<br />
(Lovely ; it is aromatic<br />
and highly stable)<br />
O<br />
C O<br />
∴ Correct option is (d).<br />
4. (c) : H +<br />
Attacks this double<br />
bond as it will give<br />
more stable carbocation<br />
+<br />
Br<br />
∴ Correct option is (c).<br />
5. (a) : H 2<br />
+ Pd will reduce double bond as well as<br />
–NO 2<br />
group.<br />
∴ Correct option is (a).<br />
6. (d) :<br />
Br –..<br />
OH oxymercuration<br />
+ Hg ( OAc)<br />
2 –<br />
–OAc<br />
+<br />
(III)<br />
(IV)<br />
Cl<br />
Cl<br />
after elimination<br />
tough to remove H<br />
from sp<br />
2 hybridised Impossible !!<br />
carbon<br />
after elimination<br />
tough as –Cl is with sp<br />
2<br />
hybridised carbon<br />
So, correct option is (b), (II).<br />
Terrible !!<br />
3. (d) : There will be the formation of a six membered<br />
ring through Diels–Alder reaction.<br />
CHEMISTRY TODAY | APRIL ‘17 67