- Page 2 and 3:
Organometallics. Paul Knochel Copyr
- Page 4 and 5:
Handbook of Functionalized Organome
- Page 6 and 7:
Contents Preface XV List of Authors
- Page 8 and 9:
Contents 3.3.9 Oxidation of Functio
- Page 10 and 11:
6.3.1 Nucleophilic Addition onto Ca
- Page 12 and 13:
9.2.3 Preparation of Functionalized
- Page 14 and 15:
13.6.4 Nickel-Catalyzed Cross-coupl
- Page 16 and 17:
Preface Since the pioneering work o
- Page 18 and 19:
XVIII List of Authors Corinne Gosmi
- Page 20 and 21:
1 Introduction Paul Knochel and Fel
- Page 22 and 23:
umorganic is directly generated in
- Page 24 and 25:
Et H 21 Et AlBu 2 + CO 2Et Cu(CN)Mg
- Page 26 and 27:
8 2 Polyfunctional Lithium Organome
- Page 28 and 29:
10 2 Polyfunctional Lithium Organom
- Page 30 and 31:
R R 12 2 Polyfunctional Lithium Org
- Page 32 and 33:
MeO MeO R 14 2 Polyfunctional Lithi
- Page 34 and 35:
N 16 2 Polyfunctional Lithium Organ
- Page 36 and 37:
18 2 Polyfunctional Lithium Organom
- Page 38 and 39:
Li LiO 20 2 Polyfunctional Lithium
- Page 40 and 41:
Li 22 2 Polyfunctional Lithium Orga
- Page 42 and 43:
24 2 Polyfunctional Lithium Organom
- Page 44 and 45:
26 Ph O Ni-Pr 2 2 Polyfunctional Li
- Page 46 and 47:
28 Li 2 Polyfunctional Lithium Orga
- Page 48 and 49:
30 2 Polyfunctional Lithium Organom
- Page 50 and 51:
Li 32 203 2 Polyfunctional Lithium
- Page 52 and 53:
34 2 Polyfunctional Lithium Organom
- Page 54 and 55:
36 Br 2 Polyfunctional Lithium Orga
- Page 56 and 57:
38 2 Polyfunctional Lithium Organom
- Page 58 and 59:
40 2 Polyfunctional Lithium Organom
- Page 60 and 61:
42 2 Polyfunctional Lithium Organom
- Page 62 and 63:
3 Functionalized Organoborane Deriv
- Page 64 and 65:
Br SSiMe 2tBu 4 3.2 Preparation and
- Page 66 and 67:
B O O 3.2 Preparation and Reaction
- Page 68 and 69:
3.2 Preparation and Reaction of Fun
- Page 70 and 71:
3.2 Preparation and Reaction of Fun
- Page 72 and 73:
Ar H Ar = O HB O CF 3 CF 3 3.2 Prep
- Page 74 and 75:
3.2 Preparation and Reaction of Fun
- Page 76 and 77:
X X Cl Cl TfO Cl 3.2 Preparation an
- Page 78 and 79:
HO HO Br OH O X N N O N 3.2 Prepara
- Page 80 and 81:
MOMO MOMO I CbzN O 3.2 Preparation
- Page 82 and 83:
3.2 Preparation and Reaction of Fun
- Page 84 and 85:
(HO) 3B O NMe 3 N N O Br 3.2 Prepar
- Page 86 and 87:
H H O O N H N H O OEt O O OEt O 3.2
- Page 88 and 89:
t BuO2C O N H 3.2 Preparation and R
- Page 90 and 91:
3.2 Preparation and Reaction of Fun
- Page 92 and 93:
3.2 Preparation and Reaction of Fun
- Page 94 and 95:
3.2 Preparation and Reaction of Fun
- Page 96 and 97:
R H2O R R B(OH) 2 B(OH) 2 3.3 Prepa
- Page 98 and 99:
3.3 Preparation and Reactions of Fu
- Page 100 and 101:
R 124 BF 3K Br 3.3 Preparation and
- Page 102 and 103:
B O O I OR O OR O O O O O OR 3.3 Pr
- Page 104 and 105:
S N O B O I O 133 3.4 Preparation a
- Page 106 and 107:
3.5 Synthesis and Reactions of Func
- Page 108 and 109:
3.7 Synthesis and Reactions of Func
- Page 110 and 111:
3.7 Synthesis and Reactions of Func
- Page 112 and 113:
3.7 Synthesis and Reactions of Func
- Page 114 and 115:
3.7 Synthesis and Reactions of Func
- Page 116 and 117:
R TrocO S N R 1 O S N 13 12 O 1 160
- Page 118 and 119:
3.7 Synthesis and Reactions of Func
- Page 120 and 121:
3.7 Synthesis and Reactions of Func
- Page 122 and 123:
22 H. Nakamura, M. Fujiwara, Y. Yam
- Page 124 and 125:
102 K. A. Scheidt, A. Tasaka, T. D.
- Page 126 and 127:
4 Polyfunctional Magnesium Organome
- Page 128 and 129:
crystallize with four-coordinated M
- Page 130 and 131:
4.2Methods of Preparation of Grigna
- Page 132 and 133:
4.2Methods of Preparation of Grigna
- Page 134 and 135:
4.2Methods of Preparation of Grigna
- Page 136 and 137:
NC Br Br 4.2Methods of Preparation
- Page 138 and 139:
N N Ph I 4.2Methods of Preparation
- Page 140 and 141:
4.2Methods of Preparation of Grigna
- Page 142 and 143:
4.2Methods of Preparation of Grigna
- Page 144 and 145:
Cl MgBr 4.2Methods of Preparation o
- Page 146 and 147:
4.2Methods of Preparation of Grigna
- Page 148 and 149:
4.2Methods of Preparation of Grigna
- Page 150 and 151:
4.2Methods of Preparation of Grigna
- Page 152 and 153:
4.2Methods of Preparation of Grigna
- Page 154 and 155:
4.2Methods of Preparation of Grigna
- Page 156 and 157:
4.2Methods of Preparation of Grigna
- Page 158 and 159:
O O O O 4.2Methods of Preparation o
- Page 160 and 161:
O Me Me O Pent I 4.2Methods of Prep
- Page 162 and 163:
4.2Methods of Preparation of Grigna
- Page 164 and 165:
4.3 Further Applications of Functio
- Page 166 and 167:
4.3 Further Applications of Functio
- Page 168 and 169:
4.3 Further Applications of Functio
- Page 170 and 171:
OTIPS I iPrMgCl OTIPS 4.3 Further A
- Page 172 and 173:
4.4 Application of Functionalized M
- Page 174 and 175:
I O O OBn Pd(t-Bu 3P) 2 (10 mol%) 4
- Page 176 and 177:
4.4 Application of Functionalized M
- Page 178 and 179:
Me OTf CO2Et + Me 4.4 Application o
- Page 180 and 181:
4.4 Application of Functionalized M
- Page 182 and 183:
12 a) F. Bickelhaupt in H. G. Riche
- Page 184 and 185:
56 G. Varchi, C. Kofink, D. M. Lind
- Page 186 and 187:
kin Trans. 1 1992, 1393; b) M. Sato
- Page 188 and 189:
31, 805; J. F. Hartwig, Angew. Chem
- Page 190 and 191:
5 Polyfunctional Silicon Organometa
- Page 192 and 193:
stereocontrol [5]. Similar chiral c
- Page 194 and 195:
5.2 Allylic Silanes seven-membered
- Page 196 and 197:
SiMe 3 33 Pr OH OSiMe 3 SiMe 3 34 O
- Page 198 and 199:
5.2 Allylic Silanes Although alkyl
- Page 200 and 201:
R O Si H 60a (R = H) 60b (R = Me) R
- Page 202 and 203:
Bpin SiMe 2Ph (CH 2) 2Ph 73 EtCH(OE
- Page 204 and 205:
BnO HO SiMe2Ph 2 BnO CHO BnO O BF3
- Page 206 and 207:
5.3 Alkenylsilanes When the same st
- Page 208 and 209:
HO I O Si Mo cat. : m n I O Si 111
- Page 210 and 211:
5.4Alkylsilanes Transition metal-ca
- Page 212 and 213:
5.4Alkylsilanes The fluoride-induce
- Page 214 and 215:
5.5 Miscellaneous Preparations and
- Page 216 and 217:
5.5 Miscellaneous Preparations and
- Page 218 and 219:
716. (c)I. E. Markó, J.-M. Planche
- Page 220 and 221:
6 Polyfunctional Tin Organometallic
- Page 222 and 223:
phine ligand or Pd II (PPh 3) 2Cl 2
- Page 224 and 225:
Bu 3 Sn Scheme 6.4 n-Pent + Me I O
- Page 226 and 227:
N N N H 2 I Scheme 6.8 N + Me Sn 3
- Page 228 and 229:
O O O NaO HO Me P O OH O OH (+)-Fos
- Page 230 and 231:
6.2 Metal-Catalyzed Coupling Reacti
- Page 232 and 233:
6.2 Metal-Catalyzed Coupling Reacti
- Page 234 and 235:
6.3 Nucleophilic Additions a-hydrox
- Page 236 and 237:
6.3 Nucleophilic Additions oxy alde
- Page 238 and 239:
6.3 Nucleophilic Additions found ap
- Page 240 and 241:
6.3 Nucleophilic Additions 6.3.1.5.
- Page 242 and 243:
6.3 Nucleophilic Additions ethylami
- Page 244 and 245:
N H 91% (ee:84%) Scheme 6.31 N CO 2
- Page 246 and 247:
6.4 Radical Reactions of Organotins
- Page 248 and 249:
TsN Scheme 6.37 + Bu 3Sn O Ph AIBN
- Page 250 and 251:
6.5.2 Tin-to-lithium Exchange 6.5.2
- Page 252 and 253:
Ar OR Scheme 6.42 N H SnBu 3 n-BuLi
- Page 254 and 255:
References 1 D. Azarian, S. S. Dua,
- Page 256 and 257:
Commun., 2002, 2608±2609; W. Su, S
- Page 258 and 259:
110 Y. Obora, M. Nakanishi, M. Toku
- Page 260 and 261:
178 Y. Yamamoto, H. Yatagai, Y. Nar
- Page 262 and 263:
J. Chem. Soc., Chem. Commun., 1995,
- Page 264 and 265:
301 D. P. G. Hamon, R. A. Massy-Wes
- Page 266 and 267:
371 I. D. Gridnev, O. L. Tok, N. A.
- Page 268 and 269:
252 7 Polyfunctional Zinc Organomet
- Page 270 and 271:
254 7 Polyfunctional Zinc Organomet
- Page 272 and 273:
256 7 Polyfunctional Zinc Organomet
- Page 274 and 275:
258 7 Polyfunctional Zinc Organomet
- Page 276 and 277:
BnO H Me 37 :1:1mixtureof diastereo
- Page 278 and 279:
262 Me 3Si 7 Polyfunctional Zinc Or
- Page 280 and 281:
264 F F 7 Polyfunctional Zinc Organ
- Page 282 and 283:
266 7 Polyfunctional Zinc Organomet
- Page 284 and 285:
Bu S O IZn(CH 2) 4ZnI 94 268 7 Poly
- Page 286 and 287:
270 7 Polyfunctional Zinc Organomet
- Page 288 and 289:
OAc MeO I EtO 2C 272 CHO S C N 7 Po
- Page 290 and 291:
274 7 Polyfunctional Zinc Organomet
- Page 292 and 293:
276 O 7 Polyfunctional Zinc Organom
- Page 294 and 295:
278 7 Polyfunctional Zinc Organomet
- Page 296 and 297:
280 E 7 Polyfunctional Zinc Organom
- Page 298 and 299:
282 MeO 2C O I 7 Polyfunctional Zin
- Page 300 and 301:
H Ph 284 7 Polyfunctional Zinc Orga
- Page 302 and 303:
286 7 Polyfunctional Zinc Organomet
- Page 304 and 305:
288 Ph N 214 O 7 Polyfunctional Zin
- Page 306 and 307:
290 7 Polyfunctional Zinc Organomet
- Page 308 and 309:
292 7 Polyfunctional Zinc Organomet
- Page 310 and 311:
294 IZn AcO 7 Polyfunctional Zinc O
- Page 312 and 313:
296 7 Polyfunctional Zinc Organomet
- Page 314 and 315:
298 7 Polyfunctional Zinc Organomet
- Page 316 and 317:
300 7 Polyfunctional Zinc Organomet
- Page 318 and 319:
302 7 Polyfunctional Zinc Organomet
- Page 320 and 321: 304 7 Polyfunctional Zinc Organomet
- Page 322 and 323: 306 7 Polyfunctional Zinc Organomet
- Page 324 and 325: 308 7 Polyfunctional Zinc Organomet
- Page 326 and 327: O 310 7 Polyfunctional Zinc Organom
- Page 328 and 329: 312 7 Polyfunctional Zinc Organomet
- Page 330 and 331: 314 7 Polyfunctional Zinc Organomet
- Page 332 and 333: 316 7 Polyfunctional Zinc Organomet
- Page 334 and 335: 318 AcO 7 Polyfunctional Zinc Organ
- Page 336 and 337: EtO 2C 320 MeO 7 Polyfunctional Zin
- Page 338 and 339: MeO O 322 n-Hept O O I Me 7 Polyfun
- Page 340 and 341: MeO 462 324 460 Br I 463 7 Polyfunc
- Page 342 and 343: 326 7 Polyfunctional Zinc Organomet
- Page 344 and 345: 328 7 Polyfunctional Zinc Organomet
- Page 346 and 347: 330 7 Polyfunctional Zinc Organomet
- Page 348 and 349: 332 7 Polyfunctional Zinc Organomet
- Page 350 and 351: 334 7 Polyfunctional Zinc Organomet
- Page 352 and 353: 336 7 Polyfunctional Zinc Organomet
- Page 354 and 355: 338 7 Polyfunctional Zinc Organomet
- Page 356 and 357: 340 7 Polyfunctional Zinc Organomet
- Page 358 and 359: 342 7 Polyfunctional Zinc Organomet
- Page 360 and 361: 344 7 Polyfunctional Zinc Organomet
- Page 362 and 363: 346 7 Polyfunctional Zinc Organomet
- Page 364 and 365: 348 8 Polyfunctional 1,1-Organodime
- Page 366 and 367: 350 O 8 Polyfunctional 1,1-Organodi
- Page 368 and 369: 352 8 Polyfunctional 1,1-Organodime
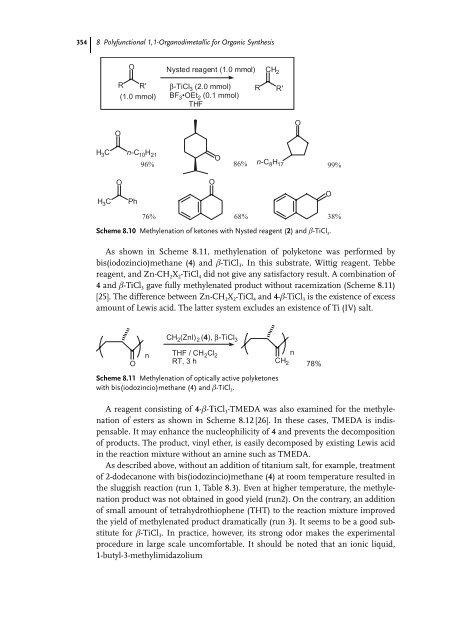
- Page 372 and 373: 356 8 Polyfunctional 1,1-Organodime
- Page 374 and 375: 358 8 Polyfunctional 1,1-Organodime
- Page 376 and 377: 360 8 Polyfunctional 1,1-Organodime
- Page 378 and 379: CH 2(ZnI) 2 4 362 8 Polyfunctional
- Page 380 and 381: 364 8 Polyfunctional 1,1-Organodime
- Page 382 and 383: 366 8 Polyfunctional 1,1-Organodime
- Page 384 and 385: 368 8 Polyfunctional 1,1-Organodime
- Page 386 and 387: 370 8 Polyfunctional 1,1-Organodime
- Page 388 and 389: 372 8 Polyfunctional 1,1-Organodime
- Page 390 and 391: 374 8 Polyfunctional 1,1-Organodime
- Page 392 and 393: 376 8 Polyfunctional 1,1-Organodime
- Page 394 and 395: 9 Polyfunctional Organocopper Reage
- Page 396 and 397: S 5 CuI·LiCl Cu(CN)Li Li naphthale
- Page 398 and 399: Br CO 2Et I CO 2Et CO 2Et Np 2CuLi
- Page 400 and 401: 9.2 Preparation of Functionalized O
- Page 402 and 403: Pent I O O Pent I CO 2Et O Br Pent
- Page 404 and 405: Ph Ph N OMe 1) n-BuLi 2) alkynylcop
- Page 406 and 407: 9.3 Applications of Functionalized
- Page 408 and 409: PrCu·MgBr 2·SMe 2 Pr Me H Pr H HO
- Page 410 and 411: 19 X. Yang, T. Rotter, C. Piazza, P
- Page 412 and 413: n-C 7H 15 398 10 Functional Organon
- Page 414 and 415: 400 10 Functional Organonickel Reag
- Page 416 and 417: 402 10 Functional Organonickel Reag
- Page 418 and 419: 404 10 Functional Organonickel Reag
- Page 420 and 421:
406 10 Functional Organonickel Reag
- Page 422 and 423:
408 10 Functional Organonickel Reag
- Page 424 and 425:
410 10 Functional Organonickel Reag
- Page 426 and 427:
412 10 Functional Organonickel Reag
- Page 428 and 429:
414 10 Functional Organonickel Reag
- Page 430 and 431:
416 10 Functional Organonickel Reag
- Page 432 and 433:
418 10 Functional Organonickel Reag
- Page 434 and 435:
420 10 Functional Organonickel Reag
- Page 436 and 437:
422 10 Functional Organonickel Reag
- Page 438 and 439:
O 424 10 Functional Organonickel Re
- Page 440 and 441:
TIPSO R 1 Cp 2ClZr O H 426 O N 10 F
- Page 442 and 443:
428 10 Functional Organonickel Reag
- Page 444 and 445:
430 10 Functional Organonickel Reag
- Page 446 and 447:
432 10 Functional Organonickel Reag
- Page 448 and 449:
434 10 Functional Organonickel Reag
- Page 450 and 451:
436 10 Functional Organonickel Reag
- Page 452 and 453:
O 438 O Br 10 Functional Organonick
- Page 454 and 455:
440 10 Functional Organonickel Reag
- Page 456 and 457:
Cl 442 CN 10 Functional Organonicke
- Page 458 and 459:
444 10 Functional Organonickel Reag
- Page 460 and 461:
446 10 Functional Organonickel Reag
- Page 462 and 463:
448 10 Functional Organonickel Reag
- Page 464 and 465:
11 Polyfunctional Metal Carbenes fo
- Page 466 and 467:
11.2 Chromium-Templated Cycloadditi
- Page 468 and 469:
11.2 Chromium-Templated Cycloadditi
- Page 470 and 471:
(CO) 5Cr O Ph 15 1) t Bu t BuOMe, 5
- Page 472 and 473:
O MeO O O O O MeO O OMe Cr(CO) 5 Me
- Page 474 and 475:
11.2.3 Cyclization of Chromium Olig
- Page 476 and 477:
(CO)5Cr OR * O S + OMe 11.2 Chromiu
- Page 478 and 479:
[2+2+1] R 1 R 1 OH (CO) 5Cr R 2 (CO
- Page 480 and 481:
11.3 Reactions of Higher Nuclearity
- Page 482 and 483:
Br O X Br O R 2 R 2 O 85: X = Si-tB
- Page 484 and 485:
11.3 Reactions of Higher Nuclearity
- Page 486 and 487:
11.4 Metathesis Reactions Catalyzed
- Page 488 and 489:
R 2 R 1 O R O 3 O 11.4 Metathesis R
- Page 490 and 491:
i Pr i Pr i Pr Me Me (R)-160 Ph O O
- Page 492 and 493:
11.5 Transmetallation The dimerizat
- Page 494 and 495:
11.6 Metal Carbenes in Peptide Chem
- Page 496 and 497:
11.7 Stereoselective Syntheses with
- Page 498 and 499:
11.7 Stereoselective Syntheses with
- Page 500 and 501:
11.7 Stereoselective Syntheses with
- Page 502 and 503:
11.7 Stereoselective Syntheses with
- Page 504 and 505:
11.7 Stereoselective Syntheses with
- Page 506 and 507:
O O O O 100% H 2N O O O O O 311a Cr
- Page 508 and 509:
11.8 Sugar Metal Carbenes as Organo
- Page 510 and 511:
References zation reactions that al
- Page 512 and 513:
35 (a) C.A. Merlic, Y. You, D.M. Mc
- Page 514 and 515:
D. R. Cefalo, P. J. Bonitatebus Jr.
- Page 516 and 517:
12 Functionalized Organozirconium a
- Page 518 and 519:
12.2 Functionalized Organozirconoce
- Page 520 and 521:
Ph O (H)ZrCp Ph O 2Cl Ph O O O 92 %
- Page 522 and 523:
12.2 Functionalized Organozirconoce
- Page 524 and 525:
i-PrO O OBu-t O H N Scheme 12.15 H
- Page 526 and 527:
BnO Scheme 12.19 O BnO + 27 28 Cp 2
- Page 528 and 529:
12.2 Functionalized Organozirconoce
- Page 530 and 531:
R 1 O O O R R1 R 2 Scheme 12.28 R P
- Page 532 and 533:
12.2 Functionalized Organozirconoce
- Page 534 and 535:
12.3 Functionalized Organotitanium
- Page 536 and 537:
12.3 Functionalized Organotitanium
- Page 538 and 539:
t-BuOOC Scheme 12.44 O CH 3 7.5 mol
- Page 540 and 541:
12.3 Functionalized Organotitanium
- Page 542 and 543:
H 13C 6 O R Scheme 12.52 SiMe 3 O T
- Page 544 and 545:
12.3 Functionalized Organotitanium
- Page 546 and 547:
O O OEt 87 Scheme 12.59 Scheme 12.6
- Page 548 and 549:
12.3 Functionalized Organotitanium
- Page 550 and 551:
12.3 Functionalized Organotitanium
- Page 552 and 553:
40 A. M. Sun, X. Huang, Heteroatom
- Page 554 and 555:
13 Manganese Organometallics for th
- Page 556 and 557:
13.2.2 Preparation of Organomangane
- Page 558 and 559:
13.3 1,2-Addition to Aldehydes and
- Page 560 and 561:
13.3.2 Manganese-Mediated Barbier-
- Page 562 and 563:
HeptMnX + HeptMnX + Scheme 13.17 Cl
- Page 564 and 565:
13.4 Preparation of Ketones by Acyl
- Page 566 and 567:
Me 3Si Cl ( ) 3 R Li 80% 82% Scheme
- Page 568 and 569:
13.5 1,4-Addition of Organomanganes
- Page 570 and 571:
BuM BuMgCl BuMnCl BuMgCl BuCu BuCu
- Page 572 and 573:
13.6 Transition-Metal-Catalyzed Cro
- Page 574 and 575:
13.6 Transition-Metal-Catalyzed Cro
- Page 576 and 577:
I Cl Scheme 13.56 MeO MnCl (1.2 equ
- Page 578 and 579:
13.7 Manganese-Mediated Cross-coupl
- Page 580 and 581:
13 C. Boucley, G. Cahiez, unpublish
- Page 582 and 583:
570 14 Polyfunctional Electrophilic
- Page 584 and 585:
572 14 Polyfunctional Electrophilic
- Page 586 and 587:
η5 η6 η4 η7 574 14 Polyfunction
- Page 588 and 589:
576 14 Polyfunctional Electrophilic
- Page 590 and 591:
578 14 Polyfunctional Electrophilic
- Page 592 and 593:
580 14 Polyfunctional Electrophilic
- Page 594 and 595:
582 14 Polyfunctional Electrophilic
- Page 596 and 597:
584 14 Polyfunctional Electrophilic
- Page 598 and 599:
586 14 Polyfunctional Electrophilic
- Page 600 and 601:
588 14 Polyfunctional Electrophilic
- Page 602 and 603:
590 14 Polyfunctional Electrophilic
- Page 604 and 605:
592 14 Polyfunctional Electrophilic
- Page 606 and 607:
51 594 CO 2Me + Fe(CO)3 CO2Me 14 Po
- Page 608 and 609:
596 14 Polyfunctional Electrophilic
- Page 610 and 611:
598 14 Polyfunctional Electrophilic
- Page 612 and 613:
Table 14.1 Examples of synthetic ap
- Page 614 and 615:
602 Entry Target molecule Disconnec
- Page 616 and 617:
Entry Target molecule Disconnection
- Page 618 and 619:
Entry Target molecule Disconnection
- Page 620 and 621:
Entry Target molecule Disconnection
- Page 622 and 623:
Target molecule Disconnections Mult
- Page 624 and 625:
612 14 Polyfunctional Electrophilic
- Page 626 and 627:
614 14 Polyfunctional Electrophilic
- Page 628 and 629:
616 14 Polyfunctional Electrophilic
- Page 630 and 631:
618 14 Polyfunctional Electrophilic
- Page 632 and 633:
620 14 Polyfunctional Electrophilic
- Page 634 and 635:
622 14 Polyfunctional Electrophilic
- Page 636 and 637:
624 14 Polyfunctional Electrophilic
- Page 638 and 639:
626 14 Polyfunctional Electrophilic
- Page 640 and 641:
15 Polyfunctional Zinc, Cobalt and
- Page 642 and 643:
Trifluoromethylzinc compounds prepa
- Page 644 and 645:
15.3 Electrochemical Synthesis and
- Page 646 and 647:
15.3.2 Carbon±Carbon Bond Formatio
- Page 648 and 649:
Cl Cl NC + MeO2C 1eq 2eq e, CoX 2 c
- Page 650 and 651:
1eq Br FG + R 2eq OAc e, CoX 2 cat
- Page 652 and 653:
Cl O + O e, FeBr 2(Bpy) n DMF, Fe a
- Page 654 and 655:
15.4 Electrosynthesis of Compounds
- Page 656 and 657:
15.4 Electrosynthesis of Compounds
- Page 658 and 659:
FG CuCN/LiCl ZnBr 0ºC CuZnBrCN 15.
- Page 660 and 661:
15.4 Electrosynthesis of Compounds
- Page 662 and 663:
15.5 General Conclusion (industrial
- Page 664 and 665:
17 Durandetti, M.; Devaud, M.; Peri
- Page 666 and 667:
I2 Index b-alkoxyalkylidenemalonic,
- Page 668 and 669:
I4 Index boronic ester 47 4-boronyl
- Page 670 and 671:
I6 Index cyclohexadiene 415 cyclohe
- Page 672 and 673:
I8 Index fluoroalkenylstannane 206
- Page 674 and 675:
I10 Index iron-catalyzed carbolithi
- Page 676 and 677:
I12 Index nickel-catalyzed carbozin
- Page 678 and 679:
I14 Index psicosecarbene complexes
- Page 680 and 681:
I16 Index Suzuki-Miyaura reaction a