Microstructural Evolution in a 17-4 PH Stainless Steel after Aging at ...
Microstructural Evolution in a 17-4 PH Stainless Steel after Aging at ...
Microstructural Evolution in a 17-4 PH Stainless Steel after Aging at ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
(a)<br />
(b)<br />
G phase Cu precipit<strong>at</strong>e<br />
020 fcc<br />
011<br />
2nm<br />
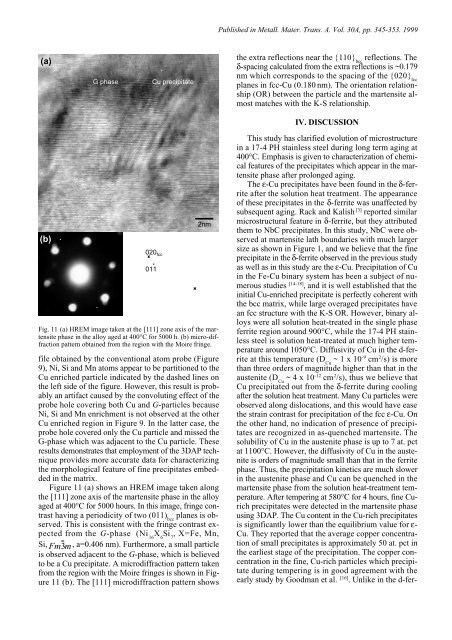
Fig. 11 (a) HREM image taken <strong>at</strong> the [111] zone axis of the martensite<br />
phase <strong>in</strong> the alloy aged <strong>at</strong> 400°C for 5000 h. (b) micro-diffraction<br />
p<strong>at</strong>tern obta<strong>in</strong>ed from the region with the Moire fr<strong>in</strong>ge.<br />
file obta<strong>in</strong>ed by the conventional <strong>at</strong>om probe (Figure<br />
9), Ni, Si and Mn <strong>at</strong>oms appear to be partitioned to the<br />
Cu enriched particle <strong>in</strong>dic<strong>at</strong>ed by the dashed l<strong>in</strong>es on<br />
the left side of the figure. However, this result is probably<br />
an artifact caused by the convolut<strong>in</strong>g effect of the<br />
probe hole cover<strong>in</strong>g both Cu and G-particles because<br />
Ni, Si and Mn enrichment is not observed <strong>at</strong> the other<br />
Cu enriched region <strong>in</strong> Figure 9. In the l<strong>at</strong>ter case, the<br />
probe hole covered only the Cu particle and missed the<br />
G-phase which was adjacent to the Cu particle. These<br />
results demonstr<strong>at</strong>es th<strong>at</strong> employment of the 3DAP technique<br />
provides more accur<strong>at</strong>e d<strong>at</strong>a for characteriz<strong>in</strong>g<br />
the morphological fe<strong>at</strong>ure of f<strong>in</strong>e precipit<strong>at</strong>es embedded<br />
<strong>in</strong> the m<strong>at</strong>rix.<br />
Figure 11 (a) shows an HREM image taken along<br />
the [111] zone axis of the martensite phase <strong>in</strong> the alloy<br />
aged <strong>at</strong> 400°C for 5000 hours. In this image, fr<strong>in</strong>ge contrast<br />
hav<strong>in</strong>g a periodicity of two (011) planes is ob-<br />
bcc<br />
served. This is consistent with the fr<strong>in</strong>ge contrast expected<br />
from the G-phase (Ni X Si , X=Fe, Mn,<br />
16 6 7<br />
Si, Fm3m , a=0.406 nm). Furthermore, a small particle<br />
is observed adjacent to the G-phase, which is believed<br />
to be a Cu precipit<strong>at</strong>e. A microdiffraction p<strong>at</strong>tern taken<br />
from the region with the Moire fr<strong>in</strong>ges is shown <strong>in</strong> Figure<br />
11 (b). The [111] microdiffraction p<strong>at</strong>tern shows<br />
Published <strong>in</strong> Metall. M<strong>at</strong>er. Trans. A. Vol. 30A, pp. 345-353. 1999<br />
the extra reflections near the {110} bcc reflections. The<br />
δ-spac<strong>in</strong>g calcul<strong>at</strong>ed from the extra reflections is ~0.<strong>17</strong>9<br />
nm which corresponds to the spac<strong>in</strong>g of the {020} fcc<br />
planes <strong>in</strong> fcc-Cu (0.180 nm). The orient<strong>at</strong>ion rel<strong>at</strong>ionship<br />
(OR) between the particle and the martensite almost<br />
m<strong>at</strong>ches with the K-S rel<strong>at</strong>ionship.<br />
IV. DISCUSSION<br />
This study has clarified evolution of microstructure<br />
<strong>in</strong> a <strong>17</strong>-4 <strong>PH</strong> sta<strong>in</strong>less steel dur<strong>in</strong>g long term ag<strong>in</strong>g <strong>at</strong><br />
400°C. Emphasis is given to characteriz<strong>at</strong>ion of chemical<br />
fe<strong>at</strong>ures of the precipit<strong>at</strong>es which appear <strong>in</strong> the martensite<br />
phase <strong>after</strong> prolonged ag<strong>in</strong>g.<br />
The ε-Cu precipit<strong>at</strong>es have been found <strong>in</strong> the δ-ferrite<br />
<strong>after</strong> the solution he<strong>at</strong> tre<strong>at</strong>ment. The appearance<br />
of these precipit<strong>at</strong>es <strong>in</strong> the δ-ferrite was unaffected by<br />
subsequent ag<strong>in</strong>g. Rack and Kalish [3] reported similar<br />
microstructural fe<strong>at</strong>ure <strong>in</strong> δ-ferrite, but they <strong>at</strong>tributed<br />
them to NbC precipit<strong>at</strong>es. In this study, NbC were observed<br />
<strong>at</strong> martensite l<strong>at</strong>h boundaries with much larger<br />
size as shown <strong>in</strong> Figure 1, and we believe th<strong>at</strong> the f<strong>in</strong>e<br />
precipit<strong>at</strong>e <strong>in</strong> the δ-ferrite observed <strong>in</strong> the previous study<br />
as well as <strong>in</strong> this study are the ε-Cu. Precipit<strong>at</strong>ion of Cu<br />
<strong>in</strong> the Fe-Cu b<strong>in</strong>ary system has been a subject of numerous<br />
studies [14-18] , and it is well established th<strong>at</strong> the<br />
<strong>in</strong>itial Cu-enriched precipit<strong>at</strong>e is perfectly coherent with<br />
the bcc m<strong>at</strong>rix, while large overaged precipit<strong>at</strong>es have<br />
an fcc structure with the K-S OR. However, b<strong>in</strong>ary alloys<br />
were all solution he<strong>at</strong>-tre<strong>at</strong>ed <strong>in</strong> the s<strong>in</strong>gle phase<br />
ferrite region around 900°C, while the <strong>17</strong>-4 <strong>PH</strong> sta<strong>in</strong>less<br />
steel is solution he<strong>at</strong>-tre<strong>at</strong>ed <strong>at</strong> much higher temper<strong>at</strong>ure<br />
around 1050°C. Diffusivity of Cu <strong>in</strong> the d-ferrite<br />
<strong>at</strong> this temper<strong>at</strong>ure (D Cu ~ 1 x 10 -9 cm 2 /s) is more<br />
than three orders of magnitude higher than th<strong>at</strong> <strong>in</strong> the<br />
austenite (D Cu ~ 4 x 10 -12 cm 2 /s), thus we believe th<strong>at</strong><br />
Cu precipit<strong>at</strong>ed out from the δ-ferrite dur<strong>in</strong>g cool<strong>in</strong>g<br />
<strong>after</strong> the solution he<strong>at</strong> tre<strong>at</strong>ment. Many Cu particles were<br />
observed along disloc<strong>at</strong>ions, and this would have ease<br />
the stra<strong>in</strong> contrast for precipit<strong>at</strong>ion of the fcc ε-Cu. On<br />
the other hand, no <strong>in</strong>dic<strong>at</strong>ion of presence of precipit<strong>at</strong>es<br />
are recognized <strong>in</strong> as-quenched martensite. The<br />
solubility of Cu <strong>in</strong> the austenite phase is up to 7 <strong>at</strong>. pct<br />
<strong>at</strong> 1100°C. However, the diffusivity of Cu <strong>in</strong> the austenite<br />
is orders of magnitude small than th<strong>at</strong> <strong>in</strong> the ferrite<br />
phase. Thus, the precipit<strong>at</strong>ion k<strong>in</strong>etics are much slower<br />
<strong>in</strong> the austenite phase and Cu can be quenched <strong>in</strong> the<br />
martensite phase from the solution he<strong>at</strong>-tre<strong>at</strong>ment temper<strong>at</strong>ure.<br />
After temper<strong>in</strong>g <strong>at</strong> 580°C for 4 hours, f<strong>in</strong>e Curich<br />
precipit<strong>at</strong>es were detected <strong>in</strong> the martensite phase<br />
us<strong>in</strong>g 3DAP. The Cu content <strong>in</strong> the Cu-rich precipit<strong>at</strong>es<br />
is significantly lower than the equilibrium value for ε-<br />
Cu. They reported th<strong>at</strong> the average copper concentr<strong>at</strong>ion<br />
of small precipit<strong>at</strong>es is approxim<strong>at</strong>ely 50 <strong>at</strong>. pct <strong>in</strong><br />
the earliest stage of the precipit<strong>at</strong>ion. The copper concentr<strong>at</strong>ion<br />
<strong>in</strong> the f<strong>in</strong>e, Cu-rich particles which precipit<strong>at</strong>e<br />
dur<strong>in</strong>g temper<strong>in</strong>g is <strong>in</strong> good agreement with the<br />
early study by Goodman et al. [16] . Unlike <strong>in</strong> the d-fer-