Medicamentos para prevenir las cefaleas migrañosas en ... - marchioli
Medicamentos para prevenir las cefaleas migrañosas en ... - marchioli
Medicamentos para prevenir las cefaleas migrañosas en ... - marchioli
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Medicam<strong>en</strong>tos</strong> <strong>para</strong> <strong>prev<strong>en</strong>ir</strong> <strong>las</strong> <strong>cefaleas</strong> migrañosas <strong>en</strong> los niños<br />
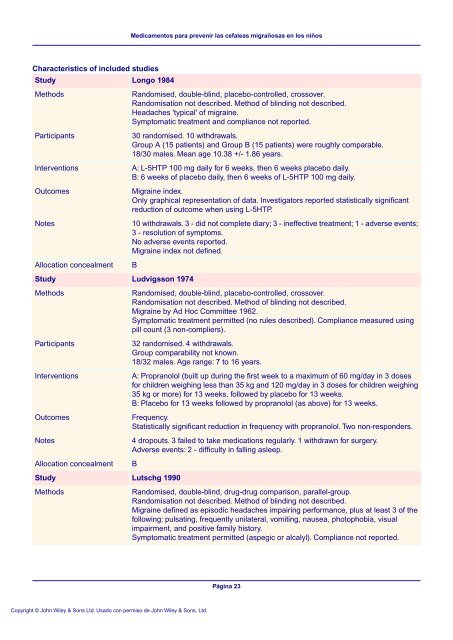
Characteristics of included studies<br />
Study<br />
Methods<br />
Participants<br />
Interv<strong>en</strong>tions<br />
Outcomes<br />
Notes<br />
Allocation concealm<strong>en</strong>t<br />
Study<br />
Methods<br />
Participants<br />
Interv<strong>en</strong>tions<br />
Outcomes<br />
Notes<br />
Allocation concealm<strong>en</strong>t<br />
Study<br />
Methods<br />
Longo 1984<br />
Randomised, double-blind, placebo-controlled, crossover.<br />
Randomisation not described. Method of blinding not described.<br />
Headaches 'typical' of migraine.<br />
Symptomatic treatm<strong>en</strong>t and compliance not reported.<br />
30 randomised. 10 withdrawals.<br />
Group A (15 pati<strong>en</strong>ts) and Group B (15 pati<strong>en</strong>ts) were roughly com<strong>para</strong>ble.<br />
18/30 males. Mean age 10.38 +/- 1.86 years.<br />
A: L-5HTP 100 mg daily for 6 weeks, th<strong>en</strong> 6 weeks placebo daily.<br />
B: 6 weeks of placebo daily, th<strong>en</strong> 6 weeks of L-5HTP 100 mg daily.<br />
Migraine index.<br />
Only graphical repres<strong>en</strong>tation of data. Investigators reported statistically significant<br />
reduction of outcome wh<strong>en</strong> using L-5HTP.<br />
10 withdrawals. 3 - did not complete diary; 3 - ineffective treatm<strong>en</strong>t; 1 - adverse ev<strong>en</strong>ts;<br />
3 - resolution of symptoms.<br />
No adverse ev<strong>en</strong>ts reported.<br />
Migraine index not defined.<br />
B<br />
Ludvigsson 1974<br />
Randomised, double-blind, placebo-controlled, crossover.<br />
Randomisation not described. Method of blinding not described.<br />
Migraine by Ad Hoc Committee 1962.<br />
Symptomatic treatm<strong>en</strong>t permitted (no rules described). Compliance measured using<br />
pill count (3 non-compliers).<br />
32 randomised. 4 withdrawals.<br />
Group com<strong>para</strong>bility not known.<br />
18/32 males. Age range: 7 to 16 years.<br />
A: Propranolol (built up during the first week to a maximum of 60 mg/day in 3 doses<br />
for childr<strong>en</strong> weighing less than 35 kg and 120 mg/day in 3 doses for childr<strong>en</strong> weighing<br />
35 kg or more) for 13 weeks, followed by placebo for 13 weeks.<br />
B: Placebo for 13 weeks followed by propranolol (as above) for 13 weeks.<br />
Frequ<strong>en</strong>cy.<br />
Statistically significant reduction in frequ<strong>en</strong>cy with propranolol. Two non-responders.<br />
4 dropouts. 3 failed to take medications regularly. 1 withdrawn for surgery.<br />
Adverse ev<strong>en</strong>ts: 2 - difficulty in falling asleep.<br />
B<br />
Lutschg 1990<br />
Randomised, double-blind, drug-drug comparison, <strong>para</strong>llel-group.<br />
Randomisation not described. Method of blinding not described.<br />
Migraine defined as episodic headaches impairing performance, plus at least 3 of the<br />
following: pulsating, frequ<strong>en</strong>tly unilateral, vomiting, nausea, photophobia, visual<br />
impairm<strong>en</strong>t, and positive family history.<br />
Symptomatic treatm<strong>en</strong>t permitted (aspegic or alcalyl). Compliance not reported.<br />
Página 23<br />
Copyright © John Wiley & Sons Ltd. Usado con permiso de John Wiley & Sons, Ltd.