bunsenmagazin - Deutsche Bunsengesellschaft für Physikalische ...
bunsenmagazin - Deutsche Bunsengesellschaft für Physikalische ...
bunsenmagazin - Deutsche Bunsengesellschaft für Physikalische ...
Sie wollen auch ein ePaper? Erhöhen Sie die Reichweite Ihrer Titel.
YUMPU macht aus Druck-PDFs automatisch weboptimierte ePaper, die Google liebt.
UNTERRICHT<br />
It is interesting to see, that the current effi ciency increases with<br />
increasing current density. Furthermore, as to be expected, the<br />
effi ciencies are independent from the substrate because after<br />
a short deposition time they are completely covered with<br />
nickel.<br />
b) electroless deposition<br />
Electroless metal deposition means the reduction of metal<br />
ions by a reducing agent that is present in the solution.<br />
Metal ion + Red ---> Metal + Ox<br />
This technique is especially advantageous when a layer of uniform<br />
thickness is desired on a material of complicated shape<br />
or when many similar objects are to be treated,<br />
The most prominent examples for such reactions are the copper<br />
deposition with formaldehyde in alkaline solution and the reduction<br />
of nickel with sodium hyphosphate. In the latter case not only<br />
nickel ions are reduced, also some hypophosphate is reduced to<br />
phosphorus. The product is a mixture of glassy nickel-phosphorus<br />
and hexagonal nickel, supersaturated with phosphorus. We<br />
will return to this reaction in the next paragraph. In order to stabilize<br />
copper ions in alkaline medium a complex former, in this<br />
case ethylendiamonotetraacetate, EDTA, must be present.<br />
In order to obtain a metal deposit only on a certain surface,<br />
it is necessary that the reaction occurs with negligible rate in<br />
the homogeneous phase and on the remaining other surfaces.<br />
This is achieved if the reduction reaction is heterogeneously<br />
catalyzed by the metal‘s surface. Hence, electroless deposition<br />
must be an autocatalytic process. If one wants to metallize an<br />
isolating surface like a polymer surface very small particles of<br />
a metallic catalyst like palladium must be deposited fi rst. The<br />
electroless deposition can easily be monitored with an EQCM.<br />
If, for instance, during the deposition a copper surface is the<br />
working electrode in an electrochemical cell, polarization leads<br />
to an external current. This current is the algebraic sum of the<br />
cathodic copper deposition current and the anodic formaldehyde<br />
oxidation current. The EQCM permits to monitor the cathodic<br />
current as a function of the potential because it is the<br />
only reaction that leads to a mass change of the electrode. The<br />
anodic current then is the difference between the total current<br />
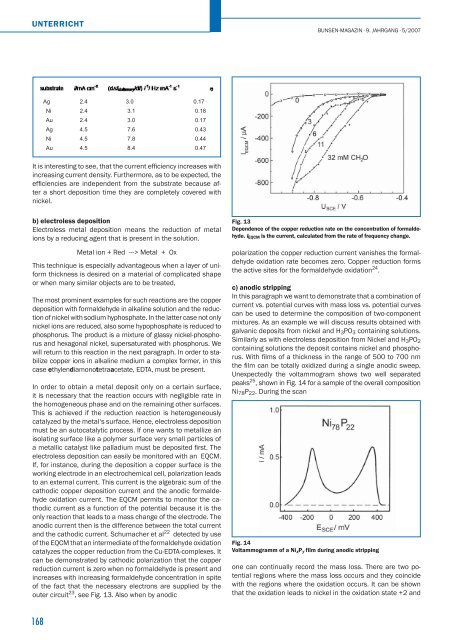
and the cathodic current. Schumacher et al 22 detected by use<br />
of the EQCM that an intermediate of the formaldehyde oxidation<br />
catalyzes the copper reduction from the Cu-EDTA-complexes. It<br />
can be demonstrated by cathodic polarization that the copper<br />
reduction current is zero when no formaldehyde is present and<br />
increases with increasing formaldehyde concentration in spite<br />
of the fact that the necessary electrons are supplied by the<br />
outer circuit 23 , see Fig. 13. Also when by anodic<br />
168<br />
Ag 2.4 3.0 0.17<br />
Ni 2.4 3.1 0.18<br />
Au 2.4 3.0 0.17<br />
Ag 4.5 7.6 0.43<br />
Ni 4.5 7.8 0.44<br />
Au 4.5 8.4 0.47<br />
BUNSEN-MAGAZIN · 9. JAHRGANG · 5/2007<br />
Fig. 13<br />
Dependence of the copper reduction rate on the concentration of formaldehyde.<br />
IEQCM is the current, calculated from the rate of frequency change.<br />
polarization the copper reduction current vanishes the formaldehyde<br />
oxidation rate becomes zero. Copper reduction forms<br />
the active sites for the formaldehyde oxidation 24 .<br />
c) anodic stripping<br />
In this paragraph we want to demonstrate that a combination of<br />
current vs. potential curves with mass loss vs. potential curves<br />
can be used to determine the composition of two-component<br />
mixtures. As an example we will discuss results obtained with<br />
galvanic deposits from nickel and H3PO3 containing solutions.<br />
Similarly as with electroless deposition from Nickel and H3PO2<br />
containing solutions the deposit contains nickel and phosphorus.<br />
With fi lms of a thickness in the range of 500 to 700 nm<br />
the fi lm can be totally oxidized during a single anodic sweep.<br />
Unexpectedly the voltammogram shows two well separated<br />
peaks 25 , shown in Fig. 14 for a sample of the overall composition<br />
Ni78P22. During the scan<br />
Fig. 14<br />
Voltammogramm of a NixPy film during anodic stripping<br />
one can continually record the mass loss. There are two potential<br />
regions where the mass loss occurs and they coincide<br />
with the regions where the oxidation occurs. It can be shown<br />
that the oxidation leads to nickel in the oxidation state +2 and