Substitution of PFOS for use in nondecorative hard chrome plating
Substitution of PFOS for use in nondecorative hard chrome plating
Substitution of PFOS for use in nondecorative hard chrome plating
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
4.3.4 F-53<br />
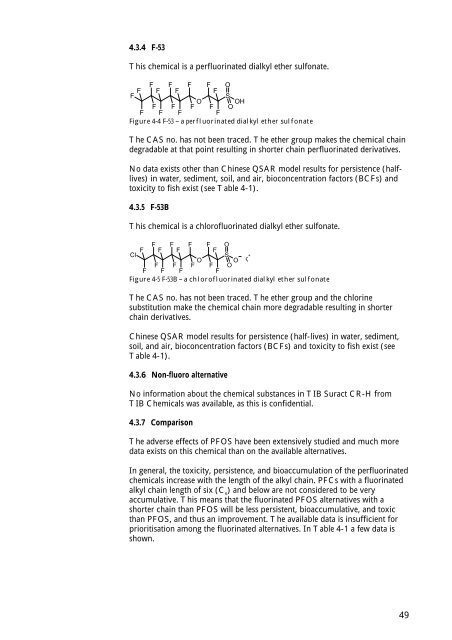
This chemical is a perfluor<strong>in</strong>ated dialkyl ether sulfonate.<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
O<br />
F<br />
F<br />
F<br />
F<br />
F<br />
O<br />
S<br />
OH<br />
O<br />
Figure 4-4 F-53 – a perfluor<strong>in</strong>ated dialkyl ether sulfonate<br />
The CAS no. has not been traced. The ether group makes the chemical cha<strong>in</strong><br />
degradable at that po<strong>in</strong>t result<strong>in</strong>g <strong>in</strong> shorter cha<strong>in</strong> perfluor<strong>in</strong>ated derivatives.<br />
No data exists other than Ch<strong>in</strong>ese QSAR model results <strong>for</strong> persistence (halflives)<br />
<strong>in</strong> water, sediment, soil, and air, bioconcentration factors (BCFs) and<br />
toxicity to fish exist (see Table 4-1).<br />
4.3.5 F-53B<br />
This chemical is a chlor<strong>of</strong>luor<strong>in</strong>ated dialkyl ether sulfonate.<br />
Cl<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
F<br />
O<br />
F<br />
F<br />
F<br />
F<br />
F<br />
O<br />
S<br />
O<br />
O<br />
K +<br />
Figure 4-5 F-53B – a chlor<strong>of</strong>luor<strong>in</strong>ated dialkyl ether sulfonate<br />
The CAS no. has not been traced. The ether group and the chlor<strong>in</strong>e<br />
substitution make the chemical cha<strong>in</strong> more degradable result<strong>in</strong>g <strong>in</strong> shorter<br />
cha<strong>in</strong> derivatives.<br />
Ch<strong>in</strong>ese QSAR model results <strong>for</strong> persistence (half-lives) <strong>in</strong> water, sediment,<br />
soil, and air, bioconcentration factors (BCFs) and toxicity to fish exist (see<br />
Table 4-1).<br />
4.3.6 Non-fluoro alternative<br />
No <strong>in</strong><strong>for</strong>mation about the chemical substances <strong>in</strong> TIB Suract CR-H from<br />
TIB Chemicals was available, as this is confidential.<br />
4.3.7 Comparison<br />
The adverse effects <strong>of</strong> <strong>PFOS</strong> have been extensively studied and much more<br />
data exists on this chemical than on the available alternatives.<br />
In general, the toxicity, persistence, and bioaccumulation <strong>of</strong> the perfluor<strong>in</strong>ated<br />
chemicals <strong>in</strong>crease with the length <strong>of</strong> the alkyl cha<strong>in</strong>. PFCs with a fluor<strong>in</strong>ated<br />
alkyl cha<strong>in</strong> length <strong>of</strong> six (C 6 ) and below are not considered to be very<br />
accumulative. This means that the fluor<strong>in</strong>ated <strong>PFOS</strong> alternatives with a<br />
shorter cha<strong>in</strong> than <strong>PFOS</strong> will be less persistent, bioaccumulative, and toxic<br />
than <strong>PFOS</strong>, and thus an improvement. The available data is <strong>in</strong>sufficient <strong>for</strong><br />
prioritisation among the fluor<strong>in</strong>ated alternatives. In Table 4-1 a few data is<br />
shown.<br />
49