PDF (Hi-Res) - Smithsonian Institution Libraries
PDF (Hi-Res) - Smithsonian Institution Libraries
PDF (Hi-Res) - Smithsonian Institution Libraries
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
NUMBER 19 97<br />
from the Maryland Academy of Sciences through<br />
the courtesy of Paul S. Watson. The specimen<br />
weighed 23.4 grams when it was received at the<br />
<strong>Smithsonian</strong> <strong>Institution</strong>. It was photographed, a<br />
model was made, and a 2.9 gram piece was removed<br />
for analysis. A polished section and two polished<br />
thin sections were prepared for optical and<br />
elecron-microprobe examination (NMNH 5423),<br />
and the remaining material was used for a bulk<br />
chemical analysis. A 19.7 gram piece was returned<br />
to the Maryland Academy of Sciences.<br />
Texture<br />
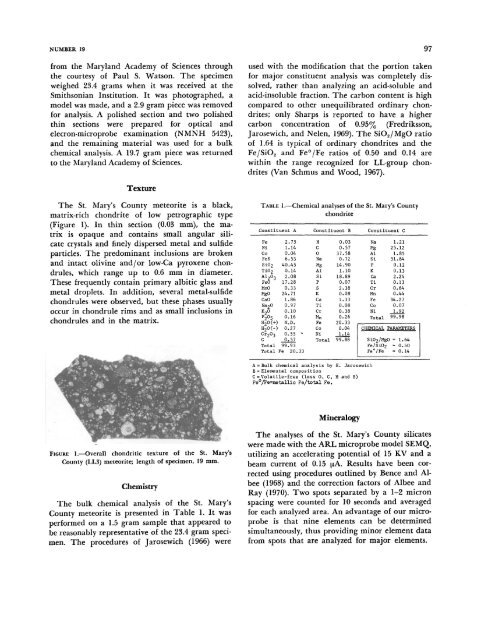
The St. Mary's County meteorite is a black,<br />
matrix-rich chondrite of low petrographic type<br />
(Figure 1). In thin section (0.03 mm), the matrix<br />
is opaque and contains small angular silicate<br />
crystals and finely dispersed metal and sulfide<br />
particles. The predominant inclusions are broken<br />
and intact olivine and/or low-Ca pyroxene chondrules,<br />
which range up to 0.6 mm in diameter.<br />
These frequently contain primary albitic glass and<br />
metal droplets. In addition, several metal-sulfide<br />
chondrules were observed, but these phases usually<br />
occur in chondrule rims and as small inclusions in<br />
chondrules and in the matrix.<br />
FIGURE 1.—Overall chondritic texture of the St. Mary's<br />
County (LL3) meteorite; length of specimen, 19 mm.<br />
Chemistry<br />
The bulk chemical analysis of the St. Mary's<br />
County meteorite is presented in Table 1. It was<br />
performed on a 1.5 gram sample that appeared to<br />
be reasonably representative of the 23.4 gram specimen.<br />
The procedures of Jarosewich (1966) were<br />
used with the modification that the portion taken<br />
for major constituent analysis was completely dissolved,<br />
rather than analyzing an acid-soluble and<br />
acid-insoluble fraction. The carbon content is high<br />
compared to other unequilibrated ordinary chondrites;<br />
only Sharps is reported to have a higher<br />
carbon concentration of 0.95% (Fredriksson,<br />
Jarosewich, and Nelen, 1969). The SiO2/MgO ratio<br />
of 1.64 is typical of ordinary chondrites and the<br />
Fe/SiO2 and Fe°/Fe ratios of 0.50 and 0.14 are<br />
within the range recognized for LL-group chondrites<br />
(Van Schmus and Wood, 1967).<br />
TABLE 1.—Chemical analyses of the St. Mary's County<br />
chondrite<br />
Constituent A<br />
Fe<br />
Ni<br />
Co<br />
FeS<br />
2.75<br />
1.14<br />
0.04<br />
6.53<br />
SiO2 40.45<br />
TiO2 0.14<br />
A12O3<br />
FeO<br />
2.08<br />
17.28<br />
MnO<br />
MgO<br />
0.33<br />
24.71<br />
CaO 1.86<br />
Na2O 0.97<br />
K2O<br />
P2O5<br />
H2O(+)<br />
H2O(-)<br />
Cr2O3<br />
C<br />
0.10<br />
0.16<br />
N.D.<br />
0.27<br />
0.55<br />
Total<br />
v<br />
0.57<br />
99.93<br />
Total Fe 20.33<br />
Constituent B<br />
H<br />
C<br />
0<br />
Na<br />
Mg<br />
Al<br />
Si<br />
P<br />
S<br />
K<br />
Ca<br />
Ti<br />
Cr<br />
Mn<br />
Fe<br />
Co<br />
Ni<br />
Total<br />
0.03<br />
0.57<br />
37.58<br />
0.72<br />
14.90<br />
1.10<br />
18.89<br />
0.07<br />
2.38<br />
0.08<br />
1.33<br />
0 = 08<br />
0.38<br />
0.26<br />
20.33<br />
0.04<br />
1.14<br />
99.88<br />
A = Bulk chemical analysis by E. Jarosewich<br />
B = Elemental composition<br />
C=Volatile-free (less 0, C, H and S)<br />
Fe°/Fe=metallic Fe/total Fe.<br />
Mineralogy<br />
Constituent C<br />
Na<br />
Mg<br />
Al<br />
Si<br />
P<br />
K<br />
Ca<br />
Ti<br />
Cr<br />
Mn<br />
Fe<br />
Co<br />
Ni<br />
Total<br />
1.21<br />
25.12<br />
1.85<br />
31.84<br />
0.12<br />
0.13<br />
2.24<br />
0.13<br />
0.64<br />
0.44<br />
34.27<br />
0.07<br />
1.92<br />
99.98<br />
CHEMICAL PARAMETERS<br />
SiO2/MgO =1.64<br />
Fe/SiO2 = 0.50<br />
Fe°/Fe = 0.14<br />
The analyses of the St. Mary's County silicates<br />
were made with the ARL microprobe model SEMQ,<br />
utilizing an accelerating potential of 15 KV and a<br />
beam current of 0.15 \iA. <strong>Res</strong>ults have been corrected<br />
using procedures outlined by Bence and Albee<br />
(1968) and the correction factors of Albee and<br />
Ray (1970). Two spots separated by a 1-2 micron<br />
spacing were counted for 10 seconds and averaged<br />
for each analyzed area. An advantage of our microprobe<br />
is that nine elements can be determined<br />
simultaneously, thus providing minor element data<br />
from spots that are analyzed for major elements.