PDF (Hi-Res) - Smithsonian Institution Libraries
PDF (Hi-Res) - Smithsonian Institution Libraries
PDF (Hi-Res) - Smithsonian Institution Libraries
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
90 SMITHSONIAN CONTRIBUTIONS TO THE EARTH SCIENCES<br />
herence is illustrated in Figure 3. The wide range<br />
of Zr/Nb ratios is somewhat surprising, since<br />
Graham and Mason (1972) found a limited range<br />
(10-32) for this ratio over a variety of meteoritic,<br />
lunar, and terrestrial materials. A geochemical coherence<br />
between Zr and Y has not previously been<br />
remarked upon to our knowledge, yet Figure 3<br />
shows that this coherence for the Allende samples<br />
is comparable to that between Zr and Hf. The<br />
cause may lie in similar condensation behavior for<br />
Zr and Y. Another possibility is that under the<br />
highly reducing conditions under which the Allende<br />
materials were formed (evidenced by the occurrence<br />
of Eu and possibly also Yb in the divalent<br />
state), Zr was trivalent, in which case its ionic radius<br />
is very close to that of Y and the two elements<br />
would tend to be closely associated geochemically.<br />
Taylor, et al. (1972) have provided evidence for the<br />
presence of trivalent zirconium in lunar rocks.<br />
Quantitative data on the platinum-group elements<br />
(Ru, Rh, Pd, Os, Ir, Pt) could not be obtained<br />
from the spark source mass spectrometer<br />
records, because of the lack of suitable standards.<br />
However, these elements were clearly present in<br />
10 20 n. 30<br />
AI2O3%<br />
Group I and Group III samples and were not seen<br />
in Group II and Group IV samples. Estimates<br />
based on relative intensities would give the following<br />
averages for Group I and Group III samples (in<br />
ppm): Pt 10; Ir 7; Os 7; Ru 8; Rh 1; Pd 1; the<br />
latter element appears to be depleted relative to<br />
the other platinum-group metals, a feature probably<br />
related to its having a considerably lower<br />
boiling point and hence being less readily condensed.<br />
Grossman (1973) has analyzed 16 Allende<br />
inclusions for Ir; all except one (which belongs to<br />
Group II from its Eu content) have Ir concentrations<br />
at 5-11 ppm. Molybdenum, a comparable siderophile<br />
element with a very high boiling point, is<br />
present in all Group I and Group III samples at<br />
a level of 4-14 ppm, is seen in two Group II samples<br />
near the lower limit of detection (0.6 ppm),<br />
and is present in Group IV samples at about 1<br />
ppm.<br />
In most samples (Table 1) uranium was close to<br />
or below the limit of detectability (0.05 ppm); however,<br />
at that level it is clearly considerably enriched<br />
over the chondritic mean (0.01 ppm). Ura-<br />
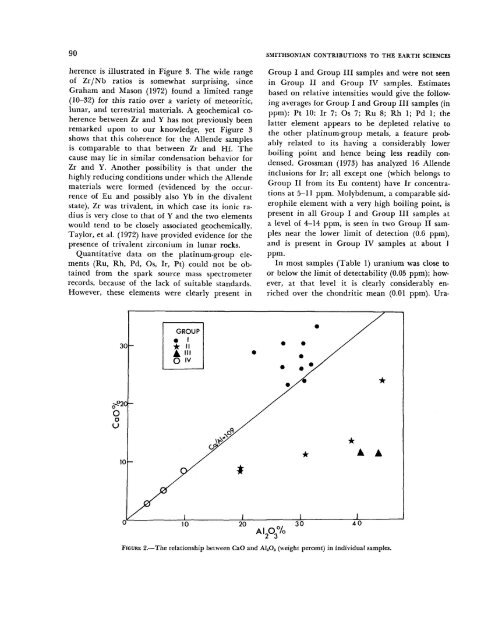
FIGURE 2.—The relationship between CaO and A12O3 (weight percent) in individual samples.<br />
40