use of tumor markers in testicular, prostate, colorectal, breast, and ...
use of tumor markers in testicular, prostate, colorectal, breast, and ...
use of tumor markers in testicular, prostate, colorectal, breast, and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
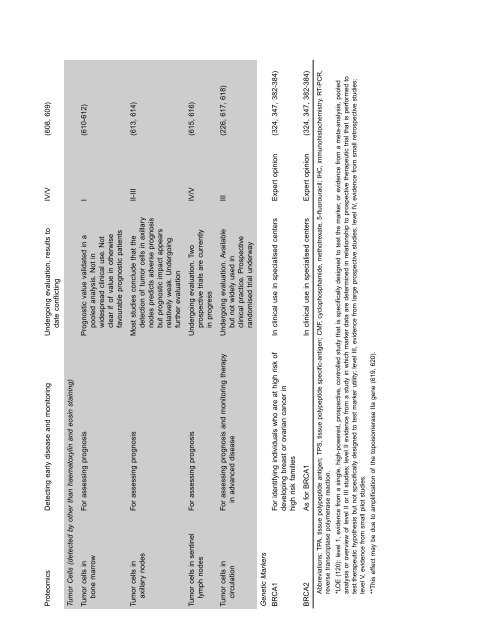
Proteomics Detect<strong>in</strong>g early disease <strong>and</strong> monitor<strong>in</strong>g Undergo<strong>in</strong>g evaluation, results to IV/V (608, 609)<br />
date conflict<strong>in</strong>g<br />
Tumor Cells (detected by other than haematoxyl<strong>in</strong> <strong>and</strong> eos<strong>in</strong> sta<strong>in</strong><strong>in</strong>g)<br />
Tumor cells <strong>in</strong> For assess<strong>in</strong>g prognosis Prognostic value validated <strong>in</strong> a I (610-612)<br />
bone marrow pooled analysis. Not <strong>in</strong><br />
widespread cl<strong>in</strong>ical <strong>use</strong>. Not<br />
clear if <strong>of</strong> value <strong>in</strong> otherwise<br />
favourable prognostic patients<br />
Tumor cells <strong>in</strong> For assess<strong>in</strong>g prognosis Most studies conclude that the II-III (613, 614)<br />
axillary nodes detection <strong>of</strong> <strong>tumor</strong> cells <strong>in</strong> axillary<br />
nodes predicts adverse prognosis<br />
but prognostic impact appears<br />
relatively weak. Undergo<strong>in</strong>g<br />
further evaluation<br />
Tumor cells <strong>in</strong> sent<strong>in</strong>el For assess<strong>in</strong>g prognosis Undergo<strong>in</strong>g evaluation. Two IV/V (615, 616)<br />
lymph nodes prospective trials are currently<br />
<strong>in</strong> progress<br />
Tumor cells <strong>in</strong> For assess<strong>in</strong>g prognosis <strong>and</strong> monitor<strong>in</strong>g therapy Undergo<strong>in</strong>g evaluation. Available III (226, 617, 618)<br />
circulation <strong>in</strong> advanced disease but not widely <strong>use</strong>d <strong>in</strong><br />
cl<strong>in</strong>ical practice. Prospective<br />
r<strong>and</strong>omised trial underway<br />
Genetic Markers<br />
BRCA1 For identify<strong>in</strong>g <strong>in</strong>dividuals who are at high risk <strong>of</strong> In cl<strong>in</strong>ical <strong>use</strong> <strong>in</strong> specialised centers Expert op<strong>in</strong>ion (324, 347, 382-384)<br />
develop<strong>in</strong>g <strong>breast</strong> or ovarian cancer <strong>in</strong><br />
high risk families<br />
BRCA2 As for BRCA1 In cl<strong>in</strong>ical <strong>use</strong> <strong>in</strong> specialised centers Expert op<strong>in</strong>ion (324, 347, 382-384)<br />
Abbreviations: TPA, tissue polypeptide antigen; TPS, tissue polypeptide specific-antigen; CMF, cyclophosphamide, methotrexate, 5-fluorouracil; IHC, immunohistochemistry. RT-PCR,<br />
reverse transcriptase polymerase reaction.<br />
*LOE (120): level 1, evidence from a s<strong>in</strong>gle, high-powered, prospective, controlled study that is specifically designed to test the marker, or evidence from a meta-analysis, pooled<br />
analysis or overview <strong>of</strong> level II or III studies; level II evidence from a study <strong>in</strong> which marker data are determ<strong>in</strong>ed <strong>in</strong> relationship to prospective therapeutic trial that is performed to<br />
test therapeutic hypothesis but not specifically designed to test marker utility; level III, evidence from large prospective studies; level IV, evidence from small retrospective studies;<br />
level V, evidence from small pilot studies.<br />
**This effect may be due to amplification <strong>of</strong> the topoisomerase IIa gene (619, 620).