use of tumor markers in testicular, prostate, colorectal, breast, and ...
use of tumor markers in testicular, prostate, colorectal, breast, and ...
use of tumor markers in testicular, prostate, colorectal, breast, and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
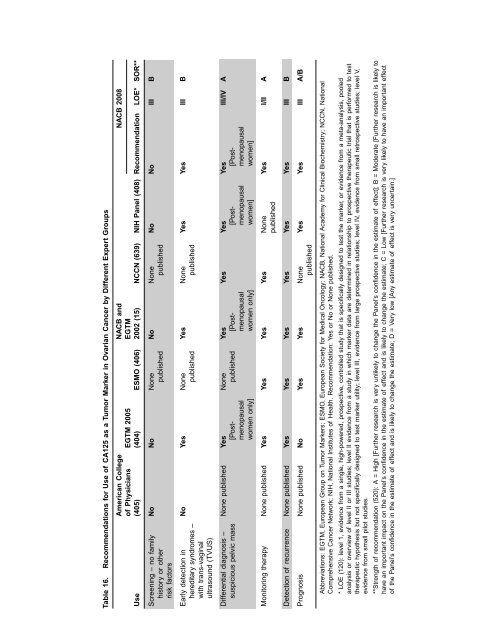
Table 16. Recommendations for Use <strong>of</strong> CA125 as a Tumor Marker <strong>in</strong> Ovarian Cancer by Different Expert Groups<br />
American College NACB <strong>and</strong><br />
NACB 2008<br />
<strong>of</strong> Physicians EGTM 2005 EGTM<br />
Use (405) (404) ESMO (406) 2002 (15) NCCN (639) NIH Panel (408) Recommendation LOE* SOR**<br />
Screen<strong>in</strong>g – no family No No None No None No No III B<br />
history or other published published<br />
risk factors<br />
Early detection <strong>in</strong> No Yes None Yes None Yes Yes III B<br />
hereditary syndromes – published published<br />
with trans-vag<strong>in</strong>al<br />
ultrasound (TVUS)<br />
Differential diagnosis – None published Yes None Yes Yes Yes Yes III/IV A<br />
suspicious pelvic mass [Post- published [Post- [Post- [Postmenopausal<br />
menopausal menopausal menopausal<br />
women only] women only] women] women]<br />
Monitor<strong>in</strong>g therapy None published Yes Yes Yes Yes None Yes I/II A<br />
published<br />
Detection <strong>of</strong> recurrence None published Yes Yes Yes Yes Yes Yes III B<br />
Prognosis None published No Yes Yes None Yes Yes III A/B<br />
published<br />
Abbreviations: EGTM, European Group on Tumor Markers; ESMO, European Society for Medical Oncology; NACB, National Academy for Cl<strong>in</strong>ical Biochemistry; NCCN, National<br />
Comprehensive Cancer Network; NIH, National Institutes <strong>of</strong> Health. Recommendation: Yes or No or None published.<br />
* LOE (120): level 1, evidence from a s<strong>in</strong>gle, high-powered, prospective, controlled study that is specifically designed to test the marker, or evidence from a meta-analysis, pooled<br />
analysis or overview <strong>of</strong> level II or III studies; level II evidence from a study <strong>in</strong> which marker data are determ<strong>in</strong>ed <strong>in</strong> relationship to prospective therapeutic trial that is performed to test<br />
therapeutic hypothesis but not specifically designed to test marker utility; level III, evidence from large prospective studies; level IV, evidence from small retrospective studies; level V,<br />
evidence from small pilot studies.<br />
**Strength <strong>of</strong> recommendation (520): A = High [Further research is very unlikely to change the Panel’s confidence <strong>in</strong> the estimate <strong>of</strong> effect]; B = Moderate [Further research is likely to<br />
have an important impact on the Panel’s confidence <strong>in</strong> the estimate <strong>of</strong> effect <strong>and</strong> is likely to change the estimate; C = Low [Further research is very likely to have an important effect<br />
<strong>of</strong> the Panel’s confidence <strong>in</strong> the estimate <strong>of</strong> effect <strong>and</strong> is likely to change the estimate; D = Very low [Any estimate <strong>of</strong> effect is very uncerta<strong>in</strong>.]