is10_sb_unit_d
is10_sb_unit_d
is10_sb_unit_d
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
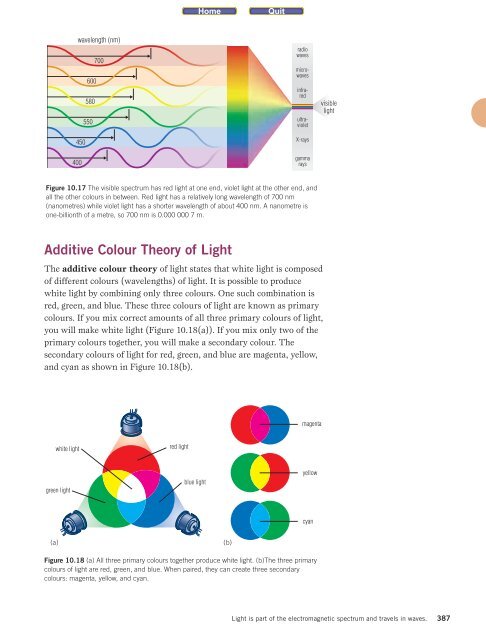
wavelength (nm)<br />
450<br />
400<br />
600<br />
580<br />
550<br />
700<br />
Additive Colour Theory of Light<br />
radio<br />
waves<br />
microwaves<br />
The additive colour theory of light states that white light is composed<br />
of different colours (wavelengths) of light. It is possible to produce<br />
white light by combining only three colours. One such combination is<br />
red, green, and blue. These three colours of light are known as primary<br />
colours. If you mix correct amounts of all three primary colours of light,<br />
you will make white light (Figure 10.18(a)). If you mix only two of the<br />
primary colours together, you will make a secondary colour. The<br />
secondary colours of light for red, green, and blue are magenta, yellow,<br />
and cyan as shown in Figure 10.18(b).<br />
infrared<br />
ultraviolet<br />
X-rays<br />
gamma<br />
rays<br />
Figure 10.17 The visible spectrum has red light at one end, violet light at the other end, and<br />
all the other colours in between. Red light has a relatively long wavelength of 700 nm<br />
(nanometres) while violet light has a shorter wavelength of about 400 nm. A nanometre is<br />
one-billionth of a metre, so 700 nm is 0.000 000 7 m.<br />
white light<br />
green light<br />
red light<br />
blue light<br />
(a) (b)<br />
magenta<br />
yellow<br />
Figure 10.18 (a) All three primary colours together produce white light. (b)The three primary<br />
colours of light are red, green, and blue. When paired, they can create three secondary<br />
colours: magenta, yellow, and cyan.<br />
cyan<br />
visible<br />
light<br />
Light is part of the electromagnetic spectrum and travels in waves.<br />
387