is10_sb_unit_d
is10_sb_unit_d
is10_sb_unit_d
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Phosphorescent Light<br />
In fluorescent lights, the phosphor emits light only while it is exposed<br />
to ultraviolet radiation. However, some substances have the ability to<br />
store energy from radiation. Phosphorescence is the ability to store<br />
the energy from a source of light and then emit it slowly over a long<br />
period. Phosphorescent materials glow in the dark for some time after<br />
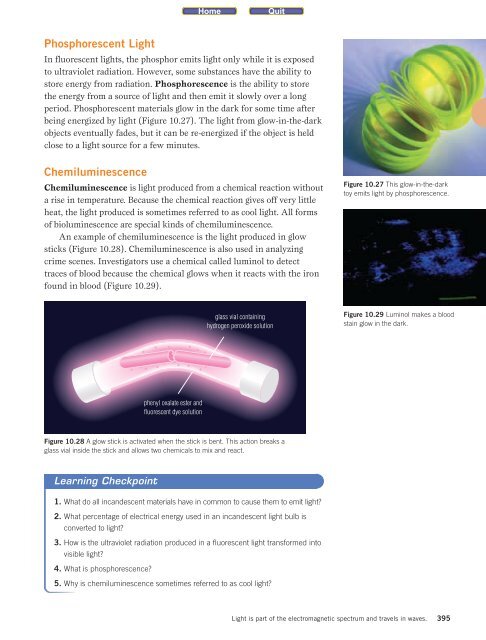
being energized by light (Figure 10.27). The light from glow-in-the-dark<br />
objects eventually fades, but it can be re-energized if the object is held<br />
close to a light source for a few minutes.<br />
Chemiluminescence<br />
Chemiluminescence is light produced from a chemical reaction without<br />
a rise in temperature. Because the chemical reaction gives off very little<br />
heat, the light produced is sometimes referred to as cool light. All forms<br />
of bioluminescence are special kinds of chemiluminescence.<br />
An example of chemiluminescence is the light produced in glow<br />
sticks (Figure 10.28). Chemiluminescence is also used in analyzing<br />
crime scenes. Investigators use a chemical called luminol to detect<br />
traces of blood because the chemical glows when it reacts with the iron<br />
found in blood (Figure 10.29).<br />
Learning Checkpoint<br />
1. What do all incandescent materials have in common to cause them to emit light?<br />
2. What percentage of electrical energy used in an incandescent light bulb is<br />
converted to light?<br />
3. How is the ultraviolet radiation produced in a fluorescent light transformed into<br />
visible light?<br />
4. What is phosphorescence?<br />
phenyl oxalate ester and<br />
fluorescent dye solution<br />
glass vial containing<br />
hydrogen peroxide solution<br />
Figure 10.28 A glow stick is activated when the stick is bent. This action breaks a<br />
glass vial inside the stick and allows two chemicals to mix and react.<br />
5. Why is chemiluminescence sometimes referred to as cool light?<br />
Figure 10.27 This glow-in-the-dark<br />
toy emits light by phosphorescence.<br />
Figure 10.29 Luminol makes a blood<br />
stain glow in the dark.<br />
Light is part of the electromagnetic spectrum and travels in waves.<br />
395