Download - Evonik Industries
Download - Evonik Industries
Download - Evonik Industries
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
10 neW or improved proCesses Category<br />
High-purity isobutene adds higher value<br />
Using methanol to convert isobutene to MTBE is an equilibrium<br />
reaction and, therefore, can also run in the opposite direction.<br />
This is where the team set to work: what kind of process could<br />
recover the chemically bound isobutene from the MTBE? How<br />
would the catalyst required for this process have to be designed?<br />
How can the byproducts of the products be removed cleanly to<br />
produce highpurity isobutene?<br />
These were crucial considerations because isobutene is a high<br />
valueadded substance with a growing market. Among other<br />
uses, it is a key raw material for methyl methacrylate, or MMA.<br />
MMA, for its part, is a highly soughtafter product used to make<br />
such products as PLEXIGLAS® for highly advanced LED flatscreen<br />
monitors, ecofriendly coatings, or even lightweight and therefore<br />
fuelsaving plastic parts in automobile production. The<br />
global market for MMA is growing by 5 percent per year. In Asia,<br />
it is growing considerably faster. Isobutene is a starting material<br />
for other chemical products such as butyl rubber (tires), adhesives,<br />
and pharmaceutical products, too.<br />
The key to success: the catalyst<br />
MTBE conversion is a heterogeneously catalyzed reaction that<br />
occurs, for best results, in the gas phase at high temperatures.<br />
The experts spent much time designing the optimal heat supply<br />
and gas flow in the reactor to ensure the highest possible<br />
conversion rate. However, the key to the success of the process<br />
is a customdesigned and highly selective catalyst. So the team<br />
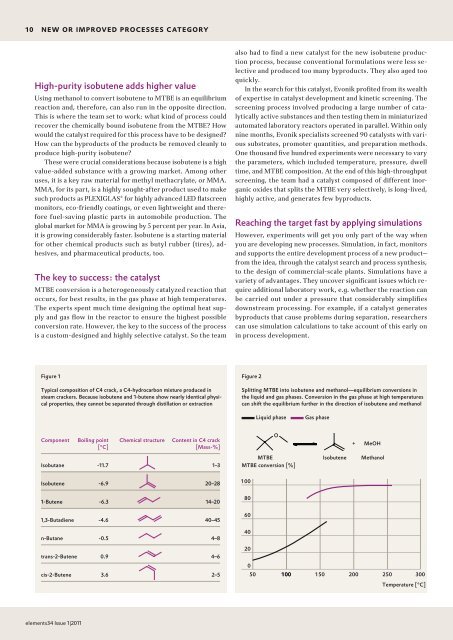
Figure 1<br />
Typical composition of C4 crack, a C4-hydrocarbon mixture produced in<br />
steam crackers. Because isobutene and 1-butene show nearly identical physical<br />
properties, they cannot be separated through distillation or extraction<br />
Component<br />
Isobutane<br />
Isobutene<br />
1-Butene<br />
1,3-Butadiene<br />
n-Butane<br />
trans-2-Butene<br />
cis-2-Butene<br />
elements34 Issue 1|2011<br />
Boiling point<br />
[°C]<br />
-11.7<br />
-6.9<br />
-6.3<br />
-4.6<br />
-0.5<br />
0.9<br />
3.6<br />
Chemical structure Content in C4 crack<br />
[Mass-%]<br />
1–3<br />
20–28<br />
14–20<br />
40–45<br />
4–8<br />
4–6<br />
2–5<br />
also had to find a new catalyst for the new isobutene production<br />
process, because conventional formulations were less selective<br />
and produced too many byproducts. They also aged too<br />
quickly.<br />
In the search for this catalyst, <strong>Evonik</strong> profited from its wealth<br />
of expertise in catalyst development and kinetic screening. The<br />
screening process involved producing a large number of catalytically<br />
active substances and then testing them in miniaturized<br />
automated laboratory reactors operated in parallel. Within only<br />
nine months, <strong>Evonik</strong> specialists screened 90 catalysts with various<br />
substrates, promoter quantities, and preparation methods.<br />
One thousand five hundred experiments were necessary to vary<br />
the parameters, which included temperature, pressure, dwell<br />
time, and MTBE composition. At the end of this highthroughput<br />
screening, the team had a catalyst composed of different inorganic<br />
oxides that splits the MTBE very selectively, is longlived,<br />
highly active, and generates few byproducts.<br />
Reaching the target fast by applying simulations<br />
However, experiments will get you only part of the way when<br />
you are developing new processes. Simulation, in fact, monitors<br />
and supports the entire development process of a new product—<br />
from the idea, through the catalyst search and process synthesis,<br />
to the design of commercialscale plants. Simulations have a<br />
variety of advantages. They uncover significant issues which require<br />
additional laboratory work, e.g. whether the reaction can<br />
be carried out under a pressure that considerably simplifies<br />
downstream processing. For example, if a catalyst generates<br />
byproducts that cause problems during separation, researchers<br />
can use simulation calculations to take account of this early on<br />
in process development.<br />
Figure 2<br />
Splitting MTBE into isobutene and methanol—equilibrium conversions in<br />
the liquid and gas phases. Conversion in the gas phase at high temperatures<br />
can shift the equilibrium further in the direction of isobutene and methanol<br />
Liquid phase Gas phase<br />
O<br />
MeOH<br />
MTBE<br />
MTBE conversion [%]<br />
Isobutene Methanol<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
50 100 150 200 250 300<br />
+<br />
Temperature [°C]