the production of thymoquinone from thymol and carvacrol
the production of thymoquinone from thymol and carvacrol
the production of thymoquinone from thymol and carvacrol
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
shared oxygen atoms with various regular arrangements, to form hundreds <strong>of</strong> different<br />
three-dimensional crystal frameworks (Kaduk et al. 1995).<br />
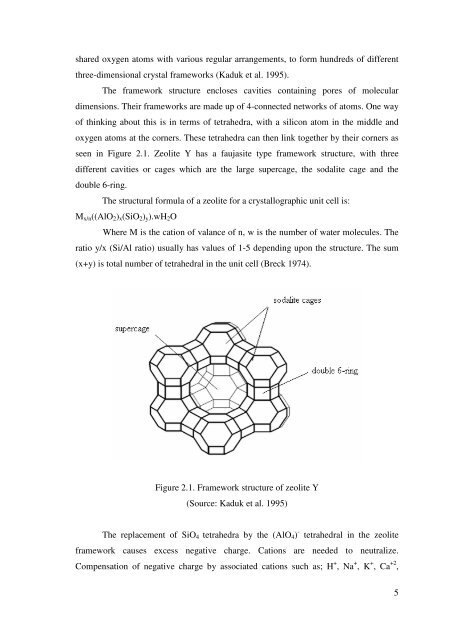
The framework structure encloses cavities containing pores <strong>of</strong> molecular<br />
dimensions. Their frameworks are made up <strong>of</strong> 4-connected networks <strong>of</strong> atoms. One way<br />
<strong>of</strong> thinking about this is in terms <strong>of</strong> tetrahedra, with a silicon atom in <strong>the</strong> middle <strong>and</strong><br />
oxygen atoms at <strong>the</strong> corners. These tetrahedra can <strong>the</strong>n link toge<strong>the</strong>r by <strong>the</strong>ir corners as<br />
seen in Figure 2.1. Zeolite Y has a faujasite type framework structure, with three<br />
different cavities or cages which are <strong>the</strong> large supercage, <strong>the</strong> sodalite cage <strong>and</strong> <strong>the</strong><br />
double 6-ring.<br />
The structural formula <strong>of</strong> a zeolite for a crystallographic unit cell is:<br />
Mx/n((AlO2)x(SiO2)y).wH2O<br />
Where M is <strong>the</strong> cation <strong>of</strong> valance <strong>of</strong> n, w is <strong>the</strong> number <strong>of</strong> water molecules. The<br />
ratio y/x (Si/Al ratio) usually has values <strong>of</strong> 1-5 depending upon <strong>the</strong> structure. The sum<br />
(x+y) is total number <strong>of</strong> tetrahedral in <strong>the</strong> unit cell (Breck 1974).<br />
Figure 2.1. Framework structure <strong>of</strong> zeolite Y<br />
(Source: Kaduk et al. 1995)<br />
The replacement <strong>of</strong> SiO4 tetrahedra by <strong>the</strong> (AlO4) - tetrahedral in <strong>the</strong> zeolite<br />
framework causes excess negative charge. Cations are needed to neutralize.<br />
Compensation <strong>of</strong> negative charge by associated cations such as; H + , Na + , K + , Ca +2 ,<br />
5