the production of thymoquinone from thymol and carvacrol
the production of thymoquinone from thymol and carvacrol
the production of thymoquinone from thymol and carvacrol
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
conversion (wt.%)<br />
45<br />
40<br />
35<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
0 100 200 300 400 500<br />
time (min.)<br />
<strong>thymol</strong> oxidation<br />
<strong>carvacrol</strong> oxidation<br />
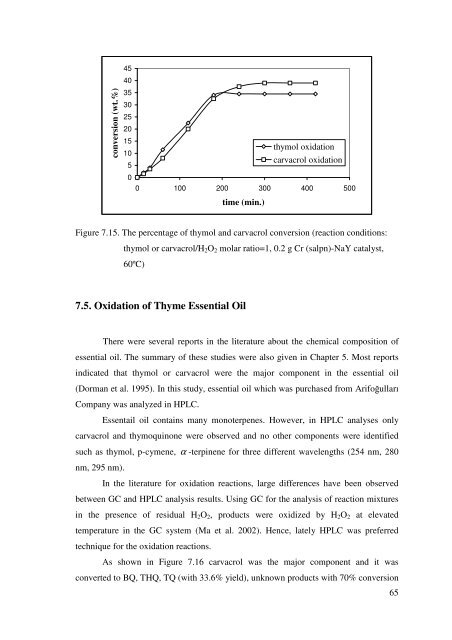
Figure 7.15. The percentage <strong>of</strong> <strong>thymol</strong> <strong>and</strong> <strong>carvacrol</strong> conversion (reaction conditions:<br />
<strong>thymol</strong> or <strong>carvacrol</strong>/H2O2 molar ratio=1, 0.2 g Cr (salpn)-NaY catalyst,<br />
60ºC)<br />
7.5. Oxidation <strong>of</strong> Thyme Essential Oil<br />
There were several reports in <strong>the</strong> literature about <strong>the</strong> chemical composition <strong>of</strong><br />
essential oil. The summary <strong>of</strong> <strong>the</strong>se studies were also given in Chapter 5. Most reports<br />
indicated that <strong>thymol</strong> or <strong>carvacrol</strong> were <strong>the</strong> major component in <strong>the</strong> essential oil<br />
(Dorman et al. 1995). In this study, essential oil which was purchased <strong>from</strong> Arifo ulları<br />
Company was analyzed in HPLC.<br />
Essentail oil contains many monoterpenes. However, in HPLC analyses only<br />
<strong>carvacrol</strong> <strong>and</strong> <strong>thymoquinone</strong> were observed <strong>and</strong> no o<strong>the</strong>r components were identified<br />
such as <strong>thymol</strong>, p-cymene, α -terpinene for three different wavelengths (254 nm, 280<br />
nm, 295 nm).<br />
In <strong>the</strong> literature for oxidation reactions, large differences have been observed<br />
between GC <strong>and</strong> HPLC analysis results. Using GC for <strong>the</strong> analysis <strong>of</strong> reaction mixtures<br />
in <strong>the</strong> presence <strong>of</strong> residual H2O2, products were oxidized by H2O2 at elevated<br />
temperature in <strong>the</strong> GC system (Ma et al. 2002). Hence, lately HPLC was preferred<br />
technique for <strong>the</strong> oxidation reactions.<br />
As shown in Figure 7.16 <strong>carvacrol</strong> was <strong>the</strong> major component <strong>and</strong> it was<br />
converted to BQ, THQ, TQ (with 33.6% yield), unknown products with 70% conversion<br />
65