the production of thymoquinone from thymol and carvacrol
the production of thymoquinone from thymol and carvacrol
the production of thymoquinone from thymol and carvacrol
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
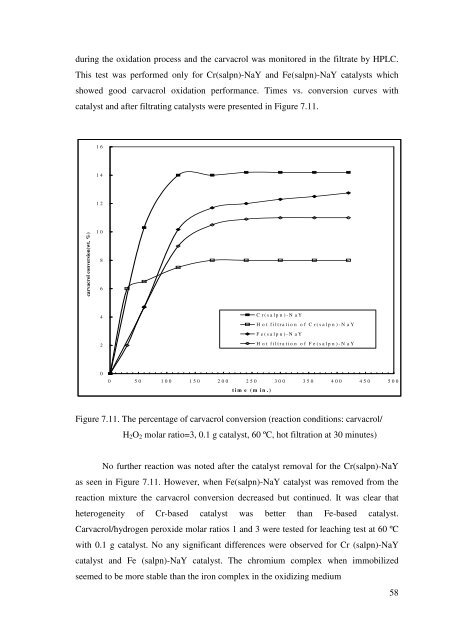
during <strong>the</strong> oxidation process <strong>and</strong> <strong>the</strong> <strong>carvacrol</strong> was monitored in <strong>the</strong> filtrate by HPLC.<br />
This test was performed only for Cr(salpn)-NaY <strong>and</strong> Fe(salpn)-NaY catalysts which<br />
showed good <strong>carvacrol</strong> oxidation performance. Times vs. conversion curves with<br />
catalyst <strong>and</strong> after filtrating catalysts were presented in Figure 7.11.<br />
<strong>carvacrol</strong> conversion(wt. %)<br />
1 6<br />
1 4<br />
1 2<br />
1 0<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 3 5 0 4 0 0 4 5 0 5 0 0<br />
t i m e ( m i n . )<br />
C r ( s a l p n ) - N a Y<br />
H o t f i l t r a t i o n o f C r ( s a l p n ) - N a Y<br />
F e ( s a l p n ) - N a Y<br />
H o t f i l t r a t i o n o f F e ( s a l p n ) - N a Y<br />
Figure 7.11. The percentage <strong>of</strong> <strong>carvacrol</strong> conversion (reaction conditions: <strong>carvacrol</strong>/<br />
H2O2 molar ratio=3, 0.1 g catalyst, 60 ºC, hot filtration at 30 minutes)<br />
No fur<strong>the</strong>r reaction was noted after <strong>the</strong> catalyst removal for <strong>the</strong> Cr(salpn)-NaY<br />
as seen in Figure 7.11. However, when Fe(salpn)-NaY catalyst was removed <strong>from</strong> <strong>the</strong><br />
reaction mixture <strong>the</strong> <strong>carvacrol</strong> conversion decreased but continued. It was clear that<br />
heterogeneity <strong>of</strong> Cr-based catalyst was better than Fe-based catalyst.<br />
Carvacrol/hydrogen peroxide molar ratios 1 <strong>and</strong> 3 were tested for leaching test at 60 ºC<br />
with 0.1 g catalyst. No any significant differences were observed for Cr (salpn)-NaY<br />
catalyst <strong>and</strong> Fe (salpn)-NaY catalyst. The chromium complex when immobilized<br />
seemed to be more stable than <strong>the</strong> iron complex in <strong>the</strong> oxidizing medium<br />
58