final .cdr
final .cdr
final .cdr
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
From the technological point of view, an ideal buccal dosage<br />
form must have three properties; It must maintains its<br />
position in the mouth for a few hours, release the drug in<br />
controlled fashion and provide drug release in a<br />
unidirectional way towards mucosa.<br />
Atenolol (beta blocker), has been widely used in the<br />
management of hypertension. The drug is well absorbed<br />
from the gastrointestinal tract but its bioavailability is low<br />
4<br />
(54%) due to extensive first pass metabolism. Since the<br />
buccal route bypasses first-pass effect, the dose of atenolol<br />
could be reduced by 50%. The physicochemical properties<br />
of atenolol, its suitable half-life (6-7 h) and low molecular<br />
weight (266.34) makes it a suitable candidate for<br />
administration by buccal route. The effective permeation of<br />
the drug through bovine buccal mucosa has already been<br />
5<br />
reported.<br />
In the present study, an attempt was made to design<br />
efficacious and prolonged release mucoadhesive tablets of<br />
atenolol using various polymers to avoid first pass<br />
metabolism, to reduce dosing frequency and to improve<br />
patient compliance with improved bioavailability.<br />
MATERIALS AND METHODS<br />
Atenolol was gifted by Rajat Pharmachem Ltd,<br />
Ankaleshwar, Gujarat. Ethyl cellulose was gifted by (Arihant<br />
Trading co., Mumbai, India), hydroxypropyl<br />
methylcellulose 15 cps, 50 cps, (Colorcon Asia Pvt. Limited,<br />
Verna, India) and carbopol 934p were gifted by ( ShinEtsu<br />
Chemical Co. Ltd Japan). All other materials were of<br />
analytical or pharmacopoeial grade and used as received.<br />
6-7<br />
Preparation of the buccal tablets<br />
Preparation: Direct compression method has been<br />
employed to prepare buccal tablets of atenolol using HPMC<br />
15cps, HPMC 50cps and Carbopol 934p as polymers.<br />
S B Shirsand et al Design and Evaluation of Atenolol Bilayer Buccal Tablets<br />
Procedure: All the ingredients including drug, polymer<br />
and excipients were weighed accurately according to the<br />
batch formula (Table 1). The drug is thoroughly mixed with<br />
mannitol on a butter paper with the help of a stainless steel<br />
spatula. Then all the ingredients except lubricant were<br />
mixed in the order of ascending weights and blended for 10<br />
min in an inflated polyethylene pouch. After uniform mixing<br />
of ingredients, lubricant was added and again mixed for 2<br />
min. The prepared blend (100 mg) of each formulation was<br />
pre-compressed, on a 10-station roatory tablet punching<br />
machine (Clit, Ahmedabad) at a pressure of 0.5 ton and<br />
turret speed of 2 rpm to form single layered flat-faced tablet<br />
of 9 mm diameter. Then, 50 mg of ethyl cellulose powder<br />
was added and <strong>final</strong> compression was done at a pressure of<br />
3.5 tons and turret speed of 2 rpm to get bilayer tablet.<br />
Evaluation of buccal tablets<br />
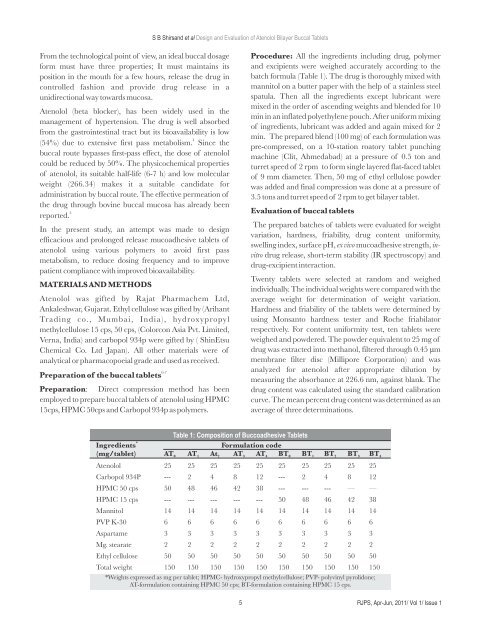
Table 1: Composition of Buccoadhesive Tablets<br />
*<br />
Ingredients Formulation code<br />
The prepared batches of tablets were evaluated for weight<br />
variation, hardness, friability, drug content uniformity,<br />
swelling index, surface pH, ex vivo mucoadhesive strength, invitro<br />
drug release, short-term stability (IR spectroscopy) and<br />
drug-excipient interaction.<br />
Twenty tablets were selected at random and weighed<br />
individually. The individual weights were compared with the<br />
average weight for determination of weight variation.<br />
Hardness and friability of the tablets were determined by<br />
using Monsanto hardness tester and Roche friabilator<br />
respectively. For content uniformity test, ten tablets were<br />
weighed and powdered. The powder equivalent to 25 mg of<br />
drug was extracted into methanol, filtered through 0.45 μm<br />
membrane filter disc (Millipore Corporation) and was<br />
analyzed for atenolol after appropriate dilution by<br />
measuring the absorbance at 226.6 nm, against blank. The<br />
drug content was calculated using the standard calibration<br />
curve. The mean percent drug content was determined as an<br />
average of three determinations.<br />
(mg/tablet) AT AT At AT AT BT BT BT BT BT<br />
0 1<br />
2 3 4 0 1 2 3 4<br />
Atenolol 25 25 25 25 25 25 25 25 25 25<br />
Carbopol 934P --- 2 4 8 12 --- 2 4 8 12<br />
HPMC 50 cps 50 48 46 42 38 --- --- --- — —<br />
HPMC 15 cps --- --- --- --- --- 50 48 46 42 38<br />
Mannitol 14 14 14 14 14 14 14 14 14 14<br />
PVP K-30 6 6 6 6 6 6 6 6 6 6<br />
Aspartame 3 3 3 3 3 3 3 3 3 3<br />
Mg. stearate 2 2 2 2 2 2 2 2 2 2<br />
Ethyl cellulose 50 50 50 50 50 50 50 50 50 50<br />
Total weight 150 150 150 150 150 150 150 150 150 150<br />
*Weights expressed as mg per tablet; HPMC- hydroxypropyl methylcellulose; PVP- polyvinyl pyrolidone;<br />
AT-formulation containing HPMC 50 cps; BT-formulation containing HPMC 15 cps.<br />
5<br />
RJPS, Apr-Jun, 2011/ Vol 1/ Issue 1