Download the full report (112 p.) - KCE
Download the full report (112 p.) - KCE
Download the full report (112 p.) - KCE
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
38 Interventions in Alzheimer’s Disease <strong>KCE</strong> Reports 111<br />
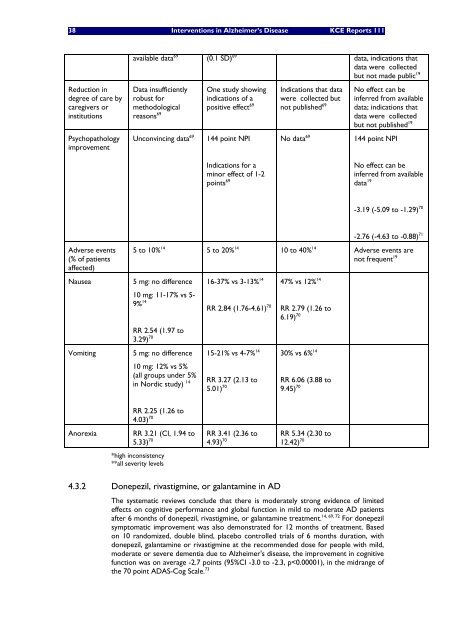
Reduction in<br />
degree of care by<br />
caregivers or<br />
institutions<br />
Psychopathology<br />
improvement<br />
Adverse events<br />
(% of patients<br />
affected)<br />
available data 69 (0.1 SD) 69 data, indications that<br />
data were collected<br />
but not made public 19<br />
Data insufficiently<br />
robust for<br />
methodological<br />
reasons 69<br />
One study showing<br />
indications of a<br />
positive effect 69<br />
Unconvincing data 69 144 point NPI<br />
Nausea 5 mg: no difference<br />
10 mg: 11-17% vs 5-<br />
9% 14<br />
Indications for a<br />
minor effect of 1-2<br />
points 69<br />
Indications that data<br />
were collected but<br />
not published 69<br />
No effect can be<br />
inferred from available<br />
data; indications that<br />
data were collected<br />
but not published 19<br />
No data 69 144 point NPI<br />
No effect can be<br />
inferred from available<br />
data 19<br />
-3.19 (-5.09 to -1.29) 70<br />
-2.76 (-4.63 to -0.88) 71<br />
5 to 10% 14 5 to 20% 14 10 to 40% 14 Adverse events are<br />
not frequent 19<br />
RR 2.54 (1.97 to<br />
3.29) 70<br />
Vomiting 5 mg: no difference<br />
10 mg: 12% vs 5%<br />
(all groups under 5%<br />
in Nordic study) 14<br />
RR 2.25 (1.26 to<br />
4.03) 70<br />
Anorexia RR 3.21 (CI, 1.94 to<br />
5.33) 70<br />
*high inconsistency<br />
**all severity levels<br />
16-37% vs 3-13% 14<br />
RR 2.84 (1.76-4.61) 70<br />
15-21% vs 4-7% 14<br />
RR 3.27 (2.13 to<br />
5.01) 70<br />
RR 3.41 (2.36 to<br />
4.93) 70<br />
4.3.2 Donepezil, rivastigmine, or galantamine in AD<br />
47% vs 12% 14<br />
RR 2.79 (1.26 to<br />
6.19) 70<br />
30% vs 6% 14<br />
RR 6.06 (3.88 to<br />
9.45) 70<br />
RR 5.34 (2.30 to<br />
12.42) 70<br />
The systematic reviews conclude that <strong>the</strong>re is moderately strong evidence of limited<br />
effects on cognitive performance and global function in mild to moderate AD patients<br />
after 6 months of donepezil, rivastigmine, or galantamine treatment. 14, 69, 72 For donepezil<br />
symptomatic improvement was also demonstrated for 12 months of treatment. Based<br />
on 10 randomized, double blind, placebo controlled trials of 6 months duration, with<br />
donepezil, galantamine or rivastigmine at <strong>the</strong> recommended dose for people with mild,<br />
moderate or severe dementia due to Alzheimer's disease, <strong>the</strong> improvement in cognitive<br />
function was on average -2.7 points (95%CI -3.0 to -2.3, p