Toxicity measurements in concentrated water samples - Rivm
Toxicity measurements in concentrated water samples - Rivm
Toxicity measurements in concentrated water samples - Rivm
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Environmental parameters<br />
As discussed, several parameters have a bear<strong>in</strong>g on extraction, which are summarized <strong>in</strong> Table 3-2. So<br />
far, no experimental concessions have been made to reduce the <strong>in</strong>fluence of environmental parameters<br />
dur<strong>in</strong>g extraction.<br />
Temperature<br />
Adsorption of hydrophobic substances <strong>in</strong>creases as the temperature rises. Adsorption of<br />
hydrophilic substances such as nonionogenic surfactants decreases. Therefore we chose to<br />
carry out the extractions at ambient temperature.<br />
Light<br />
Surface <strong>water</strong> <strong>samples</strong> usually conta<strong>in</strong> different forms of life, e.g. algae. In light, algae<br />
cont<strong>in</strong>ue primary production, result<strong>in</strong>g <strong>in</strong> an <strong>in</strong>crease <strong>in</strong> pH, which <strong>in</strong> turn can change the<br />
effectiveness of the adsorption of chemicals. Photodegradation of components may also<br />
occur. For this reason, extractions were carried out under reduced light conditions.<br />
In addition to the parameters mentioned <strong>in</strong> Table 3-2, it was also checked if the concentration of<br />
organic solvents <strong>in</strong> the <strong>water</strong> could disturb the extraction. It was found that only above a solvent<br />
concentration of 20 % (!) extraction efficiency decreased. Such values are far above values found <strong>in</strong><br />
surface <strong>water</strong>s.<br />
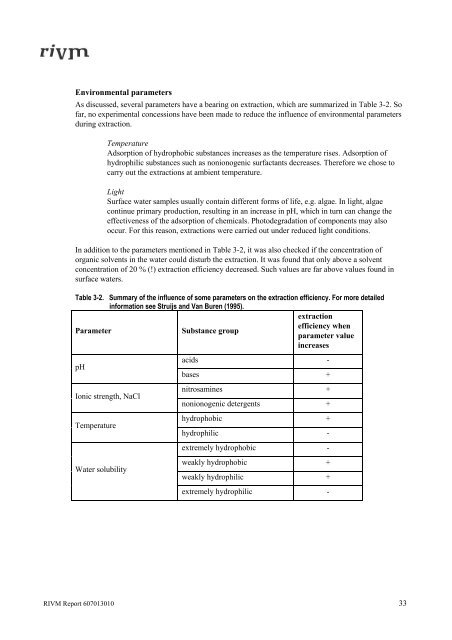
Table 3-2. Summary of the <strong>in</strong>fluence of some parameters on the extraction efficiency. For more detailed<br />
<strong>in</strong>formation see Struijs and Van Buren (1995).<br />
extraction<br />
Parameter Substance group<br />
efficiency when<br />
parameter value<br />
<strong>in</strong>creases<br />
pH<br />
Ionic strength, NaCl<br />
Temperature<br />
Water solubility<br />
acids -<br />
bases +<br />
nitrosam<strong>in</strong>es +<br />
nonionogenic detergents +<br />
hydrophobic +<br />
hydrophilic -<br />
extremely hydrophobic -<br />
weakly hydrophobic +<br />
weakly hydrophilic +<br />
extremely hydrophilic -<br />
RIVM Report 607013010 33